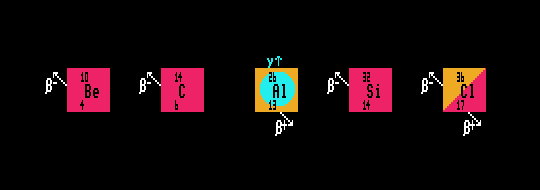
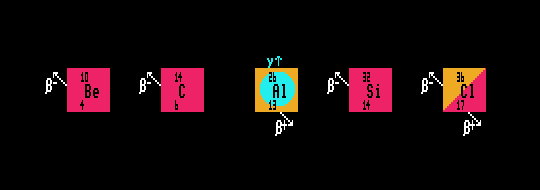
Cosmogenic Nuclides can be split into two varieties, meteoric cosmogenic nuclei produced by primary cosmic rays (mainly protons and alpha particles) interacting with atoms in the Earths' (mainly upper) atmosphere, and terrestrial cosmogenic nuclei produced by the cascade of secondary particles (mainly neutrons and muons) [which are themselves created by primary cosmic rays when they hit the upper atmosphere] interacting with atoms on the surface of Earth. Some are formed by neutron capture events, others by spallation, where a high-speed projectile knocks various components out of the targets' nucleus, leaving several differnt nuclei in its place.
The following radioactive nuclides are being continually created on Earth by cosmic ray bombardment, acting on target nuclei either in dust particles in the upper atmosphere, or to a lesser extent, on exposed rocks at the Earths surface where fewer cosmic rays are able to penetrate:
| Nuclide | Decay Mode | Halflife | Target Nuclide | Production Rate |
|---|---|---|---|---|
| iodine-129 |
|
15.7 M yrs | Te-128, Te-130, Ba | |
| manganese-53 |
|
3.7 M yrs | ||
| beryllium-10 |
|
1.39 M yrs | O-16, Si-28, Be-7, Be-9, B-10, C-13 | c. 5.2 atoms/gram/yr (in surface quartz) |
| aluminium-26 |
|
740,000 yrs | Si-28, Na-23, Mg-25 | |
| chlorine-36 |
|
301,000 yrs | Ca-40, K-39, Cl-35, S-33, Ar | 1.6g/yr (stratosphere) |
| krypton-81 | EC | 210,000 yrs | spallation or n-capture by Kr | |
| calcium-41 |
|
103,000 yrs | ||
| nickel-59 |
|
76,000 yrs | ||
| carbon-14 |
|
5730 yrs | O-16, O-17, Si-28, N-14, B-11 | 2 atoms/cm2/second in atmosphere |
| silicon-32 |
|
276 yrs | spallation from Ar | |
| argon-39 |
|
268 yrs | spallation from Ar-40? | |
| hydrogen-3 |
|
12.3 yrs | ||
| sodium-22 |
|
2.6 yrs | ||
| sulphur-35 |
|
87.5 days | spallation from Ar | |
| beryllium-7 |
EC |
53.3 days | ||
| argon-37 |
EC |
34 days | ||
| phosphorus-37 |
|
25.3 days | ||
| phosphorus-32 |
|
14.3 days | ||
| sodium-24 |
|
15 hrs |
The main nuclear processes involved in the creation of cosmogenic nuclides are spallation, muon capture (similar to electron capture) and neutron activation.
Some stable cosmogenic nuclides are also produced, for instance helium-3 and neon-21, but some of these nuclei also have other terrestrial origins. These two nuclides, and three other radioactive terrestrial cosmogenic nuclides (beryllium-10, chlorine-36 and aluminium-26) are the most commonly used cosmogenic nuclides in geological rock dating, where they accumulate within rock which overlays the Earths surface. They cannot penetrate to any great depth. Thus glacial erratics and some meteor crators can be dated this way. Any such dating is complicated by the variation in cosmic rays from year to year, decade to decade, (some cosmic rays are affected by the approximately 11-year [which varies from 8-14 years] solar sunspot cycle) and with variation in Earths' geomagnetic latitude, not to mention altitude. For instance, the beryllium-10 production rate (as shown by ice-core records) trebles during sun-spot minima, with the greatest variation being experienced at the highest latitudes (the Poles of the Earth) and the least variation at the Equator. The production rate also varies with altitude, the magnitude of Earths geomagnetic field, the angle of incidence and the sample depth.
Beryllium-10 is mostly produced by spallation from oxygen, magnesium, silicon and iron and most commonly measured in magnetite, olivine and quartz. Aluminium-26 is primarily produced by spallation from silicon, aluminium and iron and measured in olivine and quartz containing rocks. Chlorine-36 is either produced by neutron capture in chlorine-35, or in spallation from calcium and potassium. the stable helium-3 is primarily produced by spallation from oxygen, silicon, iron, magnesium, calcium or aluminium and often measured in olivine and pyroxene, but is not retained well in quartz. The non-radioactive neon-21 is produced by spallation from sodium, iron, magnesium, silicon, iron or aluminium and measured in quartz, pyroxene and olivine.
There are a large number of different cosmogenic nuclei, but only those with longish half-lives can be used for surface-exposure dating. For instance, sodium-24, with a half-life of only 15 hours, geologically speaking disappears the instant that it is formed, whilst others heavier than iron are produced in such minute amounts as to be immeasurable anyway.
NOTES ON THE ABOVE
Iodine-129 is found in tellurium ores produced by cosmic ray muon bombardment of tellurium-130.
The ratio of beryllium-10 to aluminium-26, both produced in the atmosphere by cosmic rays, is used to determine how fast atmospherically produced nuclides are carried through geological processes.
Carbon-14 (the essence of radiocarbon dating) is produced by neutron bombardment of nitrogen-14 in the atmosphere: N-14 + n ![]() (C-14 + H-1) or (C-12 + H-3).
(C-14 + H-1) or (C-12 + H-3).
Neptunium-237, halflife 2.14 Million years, is not found on Earth, but is found in moon rocks, and produced in them by the action of Cosmic Rays.
Helium-3, an extremely rare isotope on Earth due both to its low production rate and its high escape rate from the Earths upper atmosphere, is much more abundant in the surface layers of moon dust, where the abundance of titanium containing rocks, primarily ilmenite (FeTiO3) trap the helium-3 ions streaming through space driven by the solar wind. The Moon, having no atmosphere, offers no resistance to such ions and they are freely able to smash into the Moons surface un-hindered, which is not so on Earth. The helium-3 ions are spallogenic in origin, that is produced by spallation reactions where high energy cosmic rays chip bits off other atoms when they crash into them. Helium-3, like tritium, could be valuable as a fuel for nuclear fusion reactors, if hot-fusion scientists are ever able to solve the enormous problems with containment of the intensely hot plasma. [On Earth, helium-3 is present in helium-4 at 137 parts per million; and helium-4 is present in the Earths atmosphere at only 5.2 parts per million, so helium-3 is truely rare on Earth].
![]()
![]()