NEUTRON CAPTURE
Slow (thermal) neutrons may be ensnared by other nuclei in a process called neutron capture. Here the neutron number, N, increases by one unit, as does the Atomic Weight, A, but the element (proton number) remains the same. A nuclide with a deficiency of neutrons will have a higher probability of capturing a passing slow neutron and hence will have a greater thermal neutron capture cross sectional area. By capturing a neutron, a previously stable nucleus may become unstable to beta decay because of an excess of neutrons. Beta decay involves the transmutation of an element to one with higher proton number. This neutron capture process and subsequent beta decay is the means by which Uranium fuel (Z=92) in a fast breeder nuclear reactor is enriched to the higher grade Plutonium fuel (Z=94). To increase the proton number by two obviously requires more than one neutron capture and beta decay event to have occurred to the original Uranium nucleus. In a fast breeder reactor, the process of neutron capture by Uranium-238 is encouraged by slowing down the energetic neutrons emitted in the fission of Uranium-235 by a moderator, usually carbon or heavy water (D2O or HDO). The reaction then proceeds thus:
| U-238 + n | 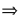 U-239 + gamma U-239 + gamma |
| U-239 |  Np-239 + electron + anti-neutrino Np-239 + electron + anti-neutrino |
| Np-239 |  Pu-239 + electron + anti-neutrino Pu-239 + electron + anti-neutrino |
A neutron absorber, in the form of control rods which are lowered in amongst the fuel rods, controls the rate of reaction in a nuclear reactor and prevents it from going critical. Boron makes good control rods, but in some reactors, ordinary water is used as the neutron absorber. If heavy water is used, the by product is tritium water, from which tritium can be extracted to make H-bombs. The 12.3 year halflife of tritium fortuitously ensures a short shelf-life for H-bombs.

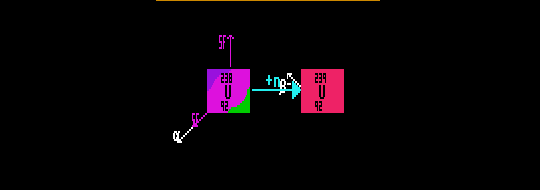
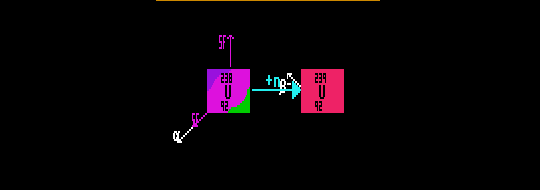
![]()