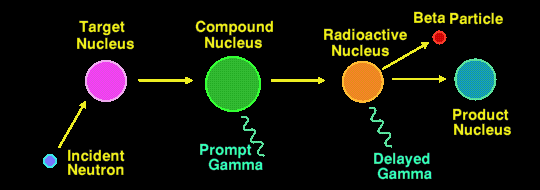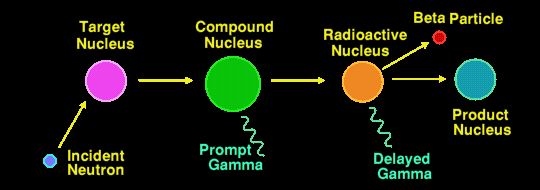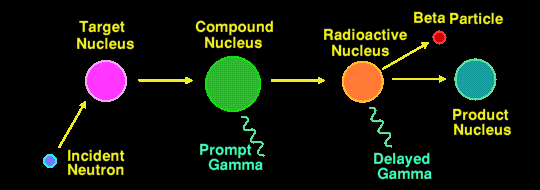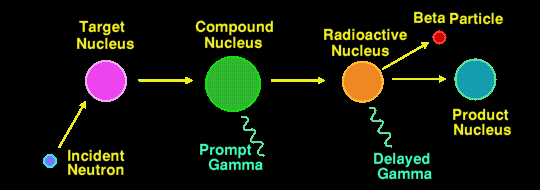

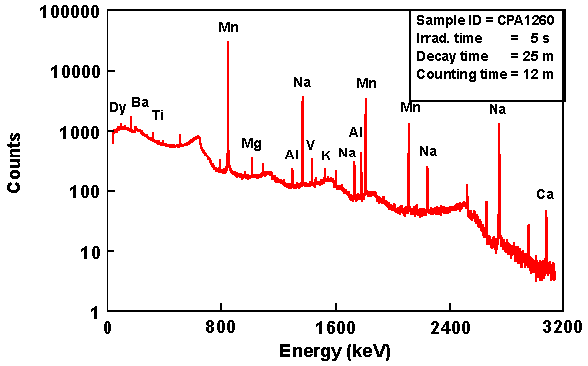
Actually, there are two methods of Neutron Activation Analysis in use, the most common method being that described above where it is the second (delayed) gamma ray that is measured. The half-lives of the nuclides that are detectable by this method may range from a few seconds to several years. Any longer and the flux of delayed gamma rays is too small to detect. The irradiation period can range from a few minutes to several days, and the integrating measurement period can range from a few seconds to half an hour or more, depending upon the elements/nuclides being measured.
The accuracy of the measurement varies from 1% to 10% of the reported value. With a neutron flux of 1x1013 per square centimetre per second, then the following is the approximate detection limit:
| Sensitivity (picograms) | E l e m e n t |
|---|---|
| 1 -10 | In, Lu, Mn |
| 10 - 100 | Au, Ho, Ir, Re, Sm, W |
| 100 - 1000 | Ag, Ar, As, Br, Cl, Co, Cs, Cu, Er, Ga, Hf, I, La, Sb, Sc, Se, Ta, Tb, Th, Tm, U, V, Yb |
| 1000 - 104 | Al, Ba, Cd, Ce, Cr, Hg, Kr, Gd, Ge, Mo, Na, Nd, Ni, Os, Pd, Rb, Rh, Ru, Sr, Te, Zn, Zr |
| 104 - 105 | Bi, Ca, K, Mg, P, Pt, Si, Sn, Ti, Tl, Xe, Y |
| 105 - 106 | F, Fe, Nb, Ne |
| 107 | Pb, S |
As you can see, the method is most sensitive to indium, lutetium and manganese, and a million times less sensitive to lead and sulphur. Neutron Activation Analysis is used in identifying artifacts such as coins and pottery from the past, or in analysing the elemental composition of tiny flecks of paint from genuine paintings in order to produce a tell-tale 'fingerprint' of artists' paintings in order that forgeries can be later identified. It is also used to inspect luggage in customs and airports in order to 'see' any suspect items within (drugs, guns, etc).
A useful portable source of neutrons is a small sample of californium-252, which is a spontaneously fissionable isotope with a fairly high probability of fission as opposed to alpha decay (branching ratio: 3.1% SF, 96.9% alpha). It has a halflife of 2.65 years, so needs replenishing fairly often. The fission products and alpha particles are blocked by a thin foil.
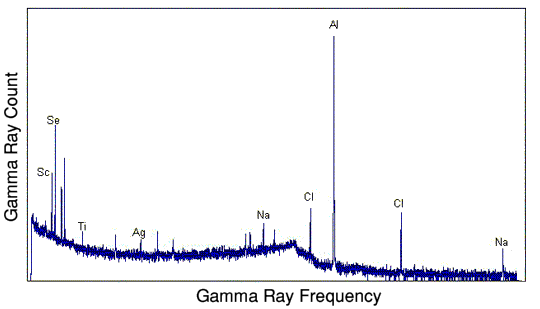
The less well used method is to measure the energies of the prompt gamma rays, those emitted by the compound nucleus. This generally requires an irradiating neutron flux a million times greater than the other method, and thus is a million times less sensitive still.
![]()
![]()