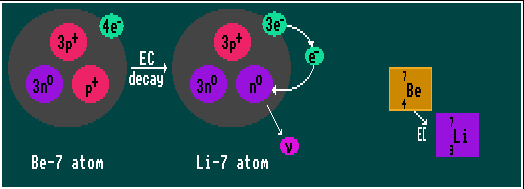
ELECTRON CAPTURE
An alternative and competing process to inverse beta decay ( decay) is Electron Capture where an internal s-orbital electron (usually from the innermost k-shell, which is why it was once called k-capture) is captured by a proton in the nucleus, changing the proton into a neutron and emitting a neutrino. Only s-orbital electrons (which have spherical orbits) can be captured, for only they traverse the nucleus; p-, d- and f-orbital electrons all avoid the nucleus. Because the electron is absorbed into the nucleus, no beta radiation is emitted. The released binding energy speeds the emitted neutrino, which emerges with one specific energy because it is a two body process only. The Atomic Weight, A, remains constant but the Atomic Number, Z, decreases by one (e.g. cobalt-57 becomes iron-57). The vacant inner-shell electron is later replaced by one from an outer shell, with the release of a photon.
decay) is Electron Capture where an internal s-orbital electron (usually from the innermost k-shell, which is why it was once called k-capture) is captured by a proton in the nucleus, changing the proton into a neutron and emitting a neutrino. Only s-orbital electrons (which have spherical orbits) can be captured, for only they traverse the nucleus; p-, d- and f-orbital electrons all avoid the nucleus. Because the electron is absorbed into the nucleus, no beta radiation is emitted. The released binding energy speeds the emitted neutrino, which emerges with one specific energy because it is a two body process only. The Atomic Weight, A, remains constant but the Atomic Number, Z, decreases by one (e.g. cobalt-57 becomes iron-57). The vacant inner-shell electron is later replaced by one from an outer shell, with the release of a photon.
Inverse beta decay is a competing process to electron capture when the atomic masses of the parent nuclide is more than two electrons lighter than the daughter nuclide. In cases where the atomic mass difference between the decaying parent atom and the daughter atom is less than two electron masses, then inverse beta decay cannot occur, but electron capture still can (provided that the daughter is lighter than the parent).
For light nuclei, inverse beta decay is favoured, but for heavier nuclei, electron capture is overwhelmingly predominant. However, in the case of inverse beta decay, an anti-electron (positron) is emitted, which can mutually annihilate with an electron producing gamma rays of 1MeV energy.
Shown is beryllium-7 decaying by electron capture into the stable lithium-7.



![]()