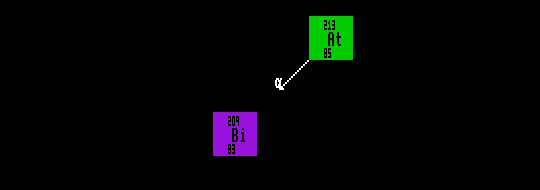

Alpha particle emission, like fission and fusion, involves barrier penetration, or the tunnelling of the alpha particle through a potential barrier. Because the width of this barrier can assume various proportions for different nuclides, the half-life for alpha decay can vary over 24 orders of magnitude from 2×1010 years for thorium-232 (which decays into radium-228) to 4.3×10-7 seconds for polonium-212 (which becomes Lead-208).
The disintegration energy (sometimes called the Q-value) liberated by alpha decay is between 2MeV and 9MeV. When the energy difference is less than 2MeV, the decay is either not observed or the halflife is incredibly long because the
The binding energy lost by the process of emitting an alpha particle is shared (in-equally) by the alpha particle and its' daughter nucleus. The alpha particle and daughter nucleus must separate with equal but opposite momenta, but because the daughter nucleus has a much greater mass than the alpha particle, the alpha particle acquires most of the energy, leaving the daughter nucleus with a slight recoil velocity (and hence energy). The energies acquired by both alpha particle and daughter nuclide are well defined.
Shown is astatine-213 decaying by alpha decay into the stable bismuth-209.
![]() -particle has to traverse a very wide energy barrier. This occurs for some nuclides with A<150 and with bismuth-209 (halflife 2×1017 years).
-particle has to traverse a very wide energy barrier. This occurs for some nuclides with A<150 and with bismuth-209 (halflife 2×1017 years).
![]()
![]()