
|
This is the relative abundance (in atom percent) of one isotope of an element in proportion to the total for that element, as occurring naturally. Some elements, such as aluminium, have only one stable isotope, aluminium-27, which represents 100% of naturally occurring aluminium, whereas others, like molybdenum or tin, have a plethora of stable isotopes representing the element. Uranium, with no stable isotopes, is nevertheless represented by three isotopes of very long half-life. Others with very short half lives, like radon, exist in equilibrium, being created by the radioactive decay of other elements with much longer half-lives. The values quoted are the average isotopic abundances for the Earth.
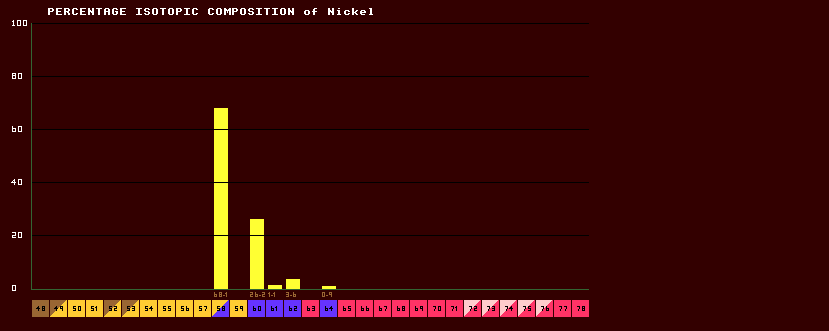
The greater the difference in isotopic weight for any given element, the greater the difference in physical properties like boiling point or melting point, and the easier it is for differential concentration of the isotopes. For instance, hydrogen has two stable isotopes, hydrogen-1 (protium) and hydrogen-2 (deuterium), and the difference in atomic weight between these two light isotopes is very large, so it is much easier for fractional distillation and separation of isotopes to take place. Sea water contains a greater proportion of heavy water [which contains deuterium] (because that is heavier and has a higher boiling point and it therefore does not evaporate as easily as light water). Therefore light water becomes slightly concentrated in rain water leaving a greater concentration of heavy water behind in the sea. Some isotopes of carbon are preferrentially absorbed by plants, but the effect is slight because the difference in atomic mass between carbon-12 to carbon-13 is small. Likewise, some isotopes of oxygen dissolve more easily in water. Thus slight differences in isotopic composition can occur in different resources. This is used to good advantage in the RatioMetric dating of ice cores and rocks. However, the difference in isotopic atomic weights between heavier elements (for instance between uranium-235 and uranium-238) is only very slight, and there is not much scope for isotopic concentration. Differences in isotopic composition can occur through other methods such as by cosmic ray bombardment of the gases in the upper atmosphere, producing new isotopes, or in the differential radioactive decay of radioactive isotopes of the same element with differing halflives. Deposits of elements having large variations in the normal isotopic composition are known as allobars. One such is the Oklo uranium allobar. In the above example, the isotopic composition of Nickel is shown. Elemental nickel comprises of a mixture of 5 'stable' (shown in blue) isotopes in the following proportions: Ni-58 composes the majority of elemental nickel, at 68.1%, with Ni-60 second at 26.2%. The other three isotopes comprise less than 5% of elemental nickel. It will be seen that the modal isotope, Ni-58, is a positron-emitter which undergoes double-inverse beta decay) but it's halflife is so long [>>80 Million years] (not shown here) that it still persists on Earth.
|
![]()