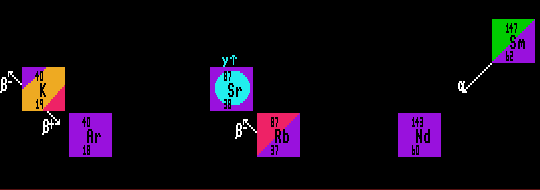
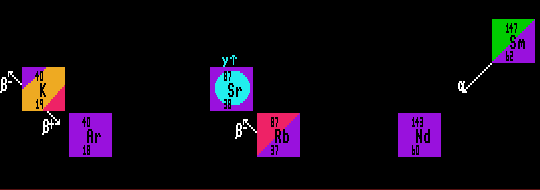
Potassium/argon dating. Potassium-40 exhibits bi-modal decay (halflife 1.277 Ga), into stable calcium-40 by beta decay, and into the stable argon-40 by inverse beta decay. Only the ratio of entrapped argon-40 to potassium-40 is useful for dating rocks; as calcium-40 is already present in most rocks, which is a pity really as this would have afforded both a method of double-checking the results and of increasing the sensitivity (because the branching ratio is 11%: argon-40 and 89%: calcium-40).
Rb-87/Sr-87. Rubidium-87, a beta decayer (halflife 48.8 Ga) becomes stable strontium-87. This method has the advantage of being resistant to invalidation by heat.
Sm-147/Nd-143. Samarium-147, an alpha decayer (halflife 108 Ga) becomes stable neodymium-143. The ratio of these two nuclides (both belonging to the rare earths with near identical chemistry) are not so easily reset (altered) by heat, so are very useful for dating metamorphic rocks.
Lu-176/Hf-176. Lutetium-176, a beta decayer (halflife 38 Ga) becomes stable hafnium-176. Both being rare earth elements with near identical chemistry, the ratio of these two nuclides is almost unaffected by the chemical history of rocks and meteorites.
Re-187/Os-187. Rhenium-187, a beta decayer (halflife 42 Ga) becomes stable osmium-187 useful for dating meteorites.
|
GROUNDWATER DATING Kr-81/Kr. Krypton-81, an electron-capture decaying isotope with a halflife of 229,000 years, can be used to date ground-water and old ice; usefully up to a million years. The Krypton-81 is produced cosmogenically in the atmosphere by the action of cosmic rays. One of the largest aquifers in the World, the freshwater Nubian aquifer, covering an area of 2 million square kilometres and between 600-1200 metres beneath the Sahara desert in Egypt has been dated at 200,000 years near the Uweinat Uplift. The measured age increases to a million years further away from the Uplift, making it one of the oldest freshwater sources found so far (March 2004). The volume of water contained within the Nubian aquifer is large, approx 50,000 cubic kilometres, the equivalent of 500 years of flow of its' source, the River Nile. A new method has beem found to efficiently count the extremely low occurrence of krypton-81 in water (about 1000 atoms per litre); the atomic-trap trace analysis method, ATTA. He-3/He-4. The ratio of the two stable helium isotopes, helium-3:helium-4 can also be used to date groundwater. The helium-4 in groundwater often increases over time due to the alpha decay of radioisotopes found in surrounding rocks, whilst the helium-3 concentration remains fixed, affording a means of dating groundwater. Cl-36/Cl. Chlorine-36, another cosmogenic nuclide, can decay by either inverse beta decay or by beta decay and has a halflife of 301,000 years. The ratio of chlorine-36 to chlorine can be used to date groundwater, and can supplement the above methods.
|
|
Other radiometric pairs include: Ar-39/Ar-40 Th-232/Pb-208 (14,100Ma) the decay series of U-235/Pb-207 (703.8Ma) and the ratios of pairs of stable isotopes of lead (eg Pb-207/Pb-206).
|
|
Also, for dating meteorites: Al-26/Mg-26 (0.75Ma) Pd-107/Ag-107 (6.5Ma) I-129/Xe-129 (15.7Ma) Pu-244/U-240 (80.5Ma).
|
PALAEOTHERMOMETRY
|
MEASURING PAST SOLAR ACTIVITYThe intensity of cosmic rays impinging on Earths atmosphere varies depending upon the activity of the Sun. Collisions between cosmic ray particles (which are mostly protons) with atmospheric oxygen nuclei create several other isotopes, amongst them both beryllium-7 (half-life 53 days) nd beryllium-10 (half-life 1.39My), which have no other presence on Earth, having long since decayed since Earth formed. These two isotopes are propelled downwards by their transferred momentum. Those that end up in either the Arctic or Antarctic get buried along with falling snow. Ice-cores drilled from this thick ice-caps thus hold a record of the Solar Activity. The two isotopes are created in known proportions, but once created, they start to decay according to their respective half-lives. By measuring the ratio of the Be-7 to Be-10 in the ice-cores, both the age of formation in the atmosphere and the activity of the Sun that produced them can be deduced. By this means the ice cores hold a dated record of past Solar Activity.
|
OTHER MEASUREMENTS USING ISOTOPIC RATIOSThe speed of light can be measured by measuring the ratios of isotopic pairs in the Oklo Uranium Allobar.
|
![]()
![]()