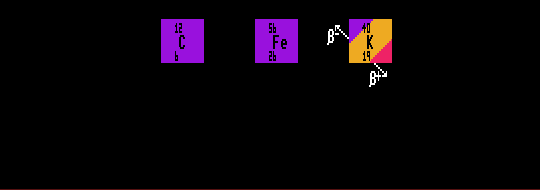

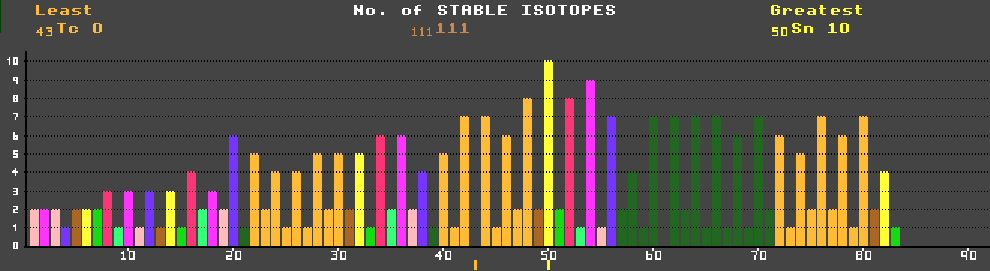
An element may exist as a mixture of several stable isotopes. The percentage abundance shows their relative distributions as found on Earth. Some elements consist of a mixture of stable and unstable isotopes when the unstable isotopes have half-lives over 80 Million years e.g. potassium-40, vanadium-50, cadmium-113, tellurium-123, samarium-148, bismuth-209 and uranium-238. Nuclei containing an even number of both nucleons (protons and neutrons) are usually stable, whilst those containing an odd complement of both are mostly unstable.
Of the stable nuclei, there are 168 examples of even/even split, whereas there are only 9 odd/odd nuclei, and these are all light nuclei. Nuclei containing 2, 8, 20, (28), 50 or 82 nucleons are particularly stable, these numbers corresponding to a closed shell of nucleons (c.f. closed electron shells of Noble gases). Shown are carbon-12, iron-56 which is the most stable isotope. Also shown is potassium-40 (which is radioactive, and therefore not stable) which can decay by either of two routes, but it's halflife is so long (1.28×109 years that it is almost stable (hence the tri-colour colour-code for that isotope: red for the beta decay, yellow for the inverse beta decay, and blue for 'stable') )
![]()