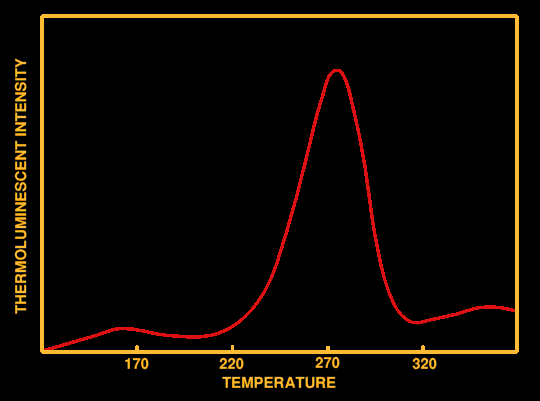
Characteristic Glow-Curve for stalagmitic calcite

Previously heated quartz and feldspar are the minerals usually investigated by thermoluminescent dating, but others such as zircon, flint and calcite have also been dated. The method is also particularly suitable for measuring the age of other archaeological remains that have undergone some process of heating or fire treatment in the past, such as pottery, ceramics, bricks, hearths, kilns, fire-pits, smelter walls, fire-cracked rocks and even industrial slag, anything that has been subject to intense heat at some time in the past. This includes volcanic materials such as lava flows, tephras, pyroclastic deposits, sediments heated by lava flows, and even meteoritic impact craters. The heating of electrically-insulating materials produces the electron-trapping defect sites necessary to store the electrons released by ionizing radiation. Since the samples are also sensitive to light, great care must be taken to keep the samples away from light, surface samples cannot be dated by this method. Naturally, to date the samples, the sample must be calibrated. This is best done by resetting the clock of part of the sample by heating it to 500 Celsius, then subjecting it to a known intensity of ionizing radiation for a certain period, an exposure, and subsequently slowly heating it to measuring the glow-curve. This is repeated with various irradiating exposures. It is then only necessary to know the intensity of ionizing radiation that the samples were originally subjected to in-situ to be able to calculate the age since the last clock-resetting event. This is usually done by measuring the present-day gamma and cosmic ray radiation at the burial site of the thermoluminescent sample. As can be imagined, the accuracy of the measured age may not be good for some specimens, but these methods can be supplemented by other dating methods such as radiometric dating to give a more accurate age. Only when these differing methods agree can any reliability be placed upon thermo-luminescence derived dates.
![]()
![]()