
Spontaneous fission involves the copious emission of energy: the difference in binding energy between the parent nucleus and the daughter products. It is unlikely that the nucleus will split into fragments of equal weight, asymmetrical splitting is favoured, with the most probable difference between the two daughter products being 45 amu, the heavy fraction lying between 130 and 145 amu (xenon to neodymium), and the light fraction between 90 to 105 amu (yttrium to ruthenium), probably due to the magic numbers of N=50 and N=82. Most of the daughters of fission are on the neutron rich side of stability and decay by beta decay. Fission, like fusion and alpha decay, is a barrier penetration (tunnelling) phenomenon.
The probability of fission increases as (Z2)/A, the fission parameter. The spontaneous fission probability of U-235 is much smaller than the probability of its alpha decay, but for the heavier californium-254 SF is the principal decay route. Fission probability is greatly increased for excited heavy nuclei.
See Induced Fission. and See Fertile Nuclides.
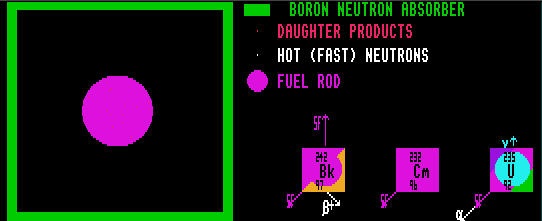
Shown is berkelium-242 (fertile isomer [SF]), curium-232 (100% SF), and uranium-235 (which can decay by alpha, or by SF).
![]()