
Because fission involves the release of two or three neutrons, the possibility of a self-sustaining chain reaction is possible if the neutron reproduction factor (gain) exceeds unity. This is attained if the mass of the fissile material exceeds a certain critical mass, which also depends upon its shape. For a spherical mass, the critical mass of U-235 is just a few kilograms.
A typical event of induced fission is shown below. Here a slow neutron is absorbed by the nucleus of a uranium-235 atom, which then fissions into barium-141 and krypton-92 plus three fast neutrons:

In a nuclear atom bomb, the mass of U-235 must be made supercritical (gain>>1) very quickly otherwise it will blow itself apart before it has all undergone fission. The induced fission of U-235 produces about 200MeV of energy per atom. This released energy is split between the various fragments and particles thus: About 80% of the total energy (165MeV) appears as the kinetic energy of the two daughter nuclides: split about 66MeV for the heavy daughter and 98MeV for the lighter daughter. Prompt neutrons (those released immediately) carry away 2MeV each, prompt gamma rays 8MeV each, and decays from radioactive daughters 26MeV, all approx.
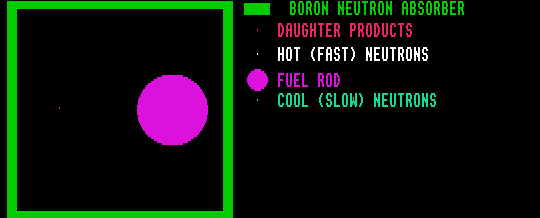
In a nuclear reactor (which uses natural uranium with an enriched U-235/U-238 ratio) the neutrons must be slowed down to thermal velocities by multiple collisions with a moderator (usually carbon or heavy water) to prevent the U-238 absorbing most of the (otherwise fast) neutrons. U-235 will preferentially absorb slow thermal neutrons, thus making a controlled chain reaction possible, with the gain held at just equal to one by control rods (usually cadmium). On absorbing a neutron, U-238 is transformed to U-239, which decays by two stages of beta decay to first Np-239 then Pu-239, which is fissile: A fast breeder reactor produces its own fuel.
For reasons of nuclear proliferation, most commercial nuclear fuel rods consist of mixed oxide fuel, containing 97% uranium oxide mixed with 3% plutonium oxide, which needs a difficult processing cycle to recover the plutonium needed for illicit nuclear bombs. The MOX fuel can only be used only for so long before fission products accumulated within it necessitate re-processing to make fresh MOX fuel rods. This process produces 3% high level waste.
Lasers can be used to induce fission. The PetaWatt laser beam at Lawrence Livermoor Laboratory has been focused on a gold foil attached in front of uranium-238. The gold instantly vaporises to a plasma, creating 100MeV electrons (the highest electron energy ever achieved by lasers). The gold plasma strikes the U-238 behind at tremendous kinetic energy temporarily fusing the gold-196 atoms with the uranium-238, which then goes on to undergo fission, typically into other isotopes around krypton-92 and barium-143, plus a few neutrons.
See Fission Isomers.
see Quantum Nucleonic Reactor
![]()
![]()