FISSION ISOMERS
FISSION ISOMERS (FERTILE NUCLIDES)
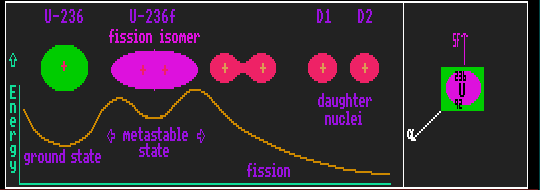
Nuclei with high atomic number (meaning a large number of protons, and therefore a high positive charge) may have two stable configurations of the nucleons. A spherical or near-spherical ground state with the least energy, and an elongated shape due to the repulsion of the large number of protons, which has a higher energy and is metastable. These are shape isomers, which differ from each other by shape, rather than spin isomers, which differ from each other by spin. This un-usual metastable state exists in so-called fission isomers, sometimes called fertile nuclides. The energy barrier on either side traps it into retaining its elongated shape since it has in-sufficient energy to surmount either barrier. The fission isomer can decay only by tunnelling through the barrier. It can do this by either of two different ways. In one way, the elongated nucleus can tunnel back into its originally more spherical ground state spin, emitting a gamma ray, just as if it were an ordinary nuclear isomer. Its isotopic identity then remains unchanged. In the other way, the elongated nucleus can tunnel itself apart, producing two separate nuclei in a process called spontaneous fission. Here its identity changes from that of itself (parent) to that of two daughter fragments of entirely different lighter elements. This mode of decay releases a great deal more binding energy than the reversion to ground state mode, which in itself, for heavy elements, is usually metastable.
In !Isotopes, fission isomers are depicted by an inscribed purple circle, and the spontaneous fission mode of decay by a purple arrow pointing upwards. Shown is uranium-236 which in its ground state is metastable and radioactive to alpha decay but which can also become excited into a higher energy metastable state, U-236f, a fission isomer.
See Fertile Nuclides. and
See Spontaneous Fission.



![]()