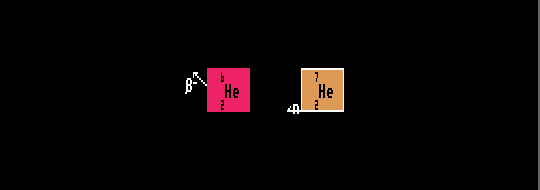
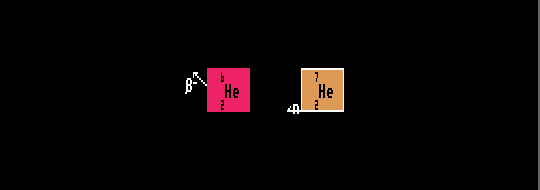
A free neutron has a rest mass of about 939.565378(21) MeV and is therefore about 1838.6866 times heavier than an electron and slightly heavier than a free proton (by 1.293332 MeV) therefore a free neutron is unstable, decaying with a half-life of about 11 minutes into a proton (+1 charge), an electron (-1 charge), and an anti-neutrino of zero charge and little or no mass. Inside a nucleus, such behaviour is called beta decay on account of the emitted beta rays (electrons). Neutrons within stable nuclides undergo no such decay, being stabilised by the (negative) binding energy. But nuclides with an excess of neutrons are liable to undergo beta decay, and in the extreme case when on the 'neutron drip line', to drip neutrons. The Segre chart does not extend below the neutron drip line, where no more neutrons can be added to the isotopes. The neutron drip line will be in-accessible to experiments for some time to come, because it is impossible to produce the heavy elements that by fragmentation could reach the limit of neutron excess. The same is not true for protons, the proton drip line has now been defined experimentally between Z=50 and Z=82 (AD 2003).
A neutron consists of three quarks, two down quarks which have a mass of 4.8 Mev each and one lighter up quark with a mass of 2.3MeV making a total mass due to the quarks only of just 11.9 MeV, a very small fraction of the protons mass. Like all fundamental particles, the mass of the quarks is derived from their interaction with the all-pervasive Higgs field which is provided by the Higgs boson(s). Most of the mass of the hadrons like (protons and neutrons and mesons) comes from not the mass of the constituent quarks and gluons themselves, but from the zero-point quantum fluctuation energy of the confined quarks and gluons; the colour force between quarks and gluons which is continually exchanging their colours, which have nothing to do with the Higgs field, it contributes but a very small fraction to the mass of a Neutron.
There are no stable isotones for N=19, 21, 35, 39, 45, 61, 71, 89, 115, and 123, all odd numbers. See the Segre Chart [POP]. Neutrons are emitted by heavy nuclides undergoing spontaneous fission: when a heavier nuclide (containing proportionately more neutrons) splits into two lighter nuclides (containing proportionally fewer neutrons), there is a surplus of neutrons. Slow moving neutrons (thermal neutrons) are able to be captured by some nuclei, whereas fast neutrons may just split the target nucleus apart (induced fission).
Ultra-cold neutrons, those with an energy less than 10-7eV, are totally reflected by most substances and are able to be confined within a bottle for a long enough time to accurately measure the halflife, 887.6 seconds. They can travel several hundred miles through superfluid liquid helium-4 unhindered, but are absorbed by the nuclei of any helium-3 atoms (transmuting them into helium-4).
Shown is Helium-7, on the neutron drip line, decaying into Helium-6 by the emission of a neutron.
For the internal structure of a neutron, see Baryons & Quarks
See also See Element Zero.
![]()