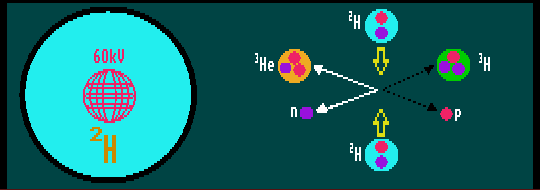

The last is, surprisingly, the most attractive. A portable fusion reactor no bigger than a football has been produced that will generate between 107 to 109 neutrons per second, not enough for neutron therapy which requires 1016, but enough for neutron activated analysis. Its main advantage being that, unlike all other sources, it produces no harmful radioactive by-products, and unlike fission sources, can be switched on and off at will. A spherical container holds deuterium gas. At its centre is a spherical wire cage the size of a tennis ball, charged to 60kV. This ionizes the deuterium atoms accelerating them towards the centre where, due to the geometry of the wire cage, they are focussed to collide. A few undergo fusion, producing either neutrons and harmless helium-3 + 3.27 MeV energy, or traces of protium and the dangerously radioactive tritium. This fusion generator cannot be used for power generation as it consumes far more energy than it would generate. The high-energy neutrons are emitted in all directions. A carefully crafted shield of boron-10, an efficient absorber of neutrons, can limit them to one direction.
A new radiation shield 60 times more effective at absorbing radiation than boron-containing steels is a nickel-based alloy containing gadolinium. Gadolinium has a very high thermal-neutron cross-section, and absorbs these neutrons very effectively.
Neutron activated analysis is a fast, sensitive and powerful technique for identifying the constituent atoms in substances. Atoms bombarded by fast neutrons are raised into excited states from where they quickly decay emitting a gamma ray of unique energy signature, from which the identity of the original atoms can be inferred. This is of use in mining, archaeology, airport bomb detectors etc.
![]()