ISOTONES
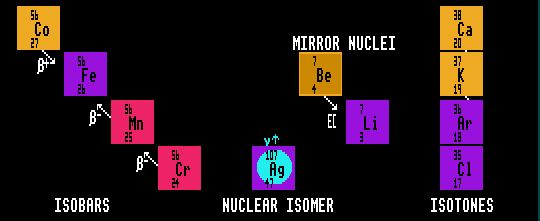
ISOBARS
Isobars are nuclei of the same Atomic Mass, A, (i.e. same number of nucleons), but differing proton number or neutron number. Thus, cobalt-56 (27 protons, 29 neutrons) is isobaric with iron-56 (26 protons 30 neutrons), manganese-56 (25p 31n) and chromium-56 (24p 32n). Isobaric means having the same number of baryons, (Protons plus Neutrons). On the Segre chart, the isobars lie on a 45 degree diagonal strip thus \ across the narrow width of the chart.
NUCLEAR ISOMERS
An isomer is any nuclide that is in an excited state, able to decay by Internal Transition (IT) to a lower energy state, by emitting a gamma ray with a halflife LONGER than 1 microsecond, by definition. This duration was chosen arbitrarily; excited nuclides can and do emit gamma radiation after any time delay. No transmutation into a different element occurs. Silver-107 is ordinarily stable, but can exist in an excited state.
MIRROR NUCLEI
An isobaric pair of nuclei in which the number of neutrons in one is equal to the number of protons in the other. Thus lithium-7 (3p 4n) is a mirror nucleus of beryllium-7 (4p 3n).
ISOTONES
IsotoNes are nuclides with the same number of neutrons, N, but differing proton number, Z. That is, they are differing elements but with the same number of neutrons. Thus, calcium-42 (20p 22n) is an isotone of potassium-41 (19p 22n), argon-40 (18p 22n) and chlorine-39 (17p 22n). On the Segre chart, the isotones lie in a vertical strip thus |.



![]()