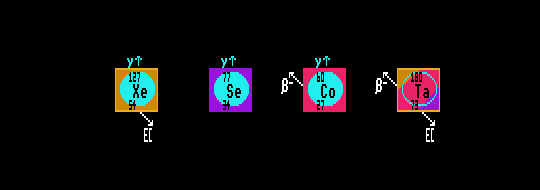
GAMMA RADIOACTIVITY
GAMMA RADIOACTIVITY by I.T. (Internal Transition)
Some nuclides can exist in higher energy excited metastable states (nuclear isomers), in which the internal arrangements of protons and neutrons in the nucleus is not in it's most stable state. This may be due to excess nuclear spin left in a daughter nuclide after its' parent has undergone radioactive decay. Such a nucleus may be non-spherical, being either prolate or oblate. The nuclide may then undergo internal re-arrangement from the higher energy state to a lower energy state losing energy as it does so by emitting a gamma ray photon. Note that in this particular kind of radioactive decay, no change in identity is involved, the nuclide remains the same throughout. Usually there exist a great many excited states of differing energy levels, therefore a whole spectrum of gamma ray emissions is possible. When the energy difference between levels exceeds the total rest mass of an electron plus positron, about 1MeV, then electron/positron pair creation can occur in competition to gamma ray emission. Even otherwise stable nuclides may exist in these excited unstable states. It is also possible for nuclides to emit gamma rays by other processes, like Internal Bremsstrahlung (IB), by relinquishing excess nuclear spin, or by internal conversion.
Tantalum-180 is unusual in that in its ground state it is unstable with a halflife of just 8.15 hours, but it has a natural occurrence on Earth and comprises 0.012% of tantalum. It is present in its metastable state, which has a halflife of 1.2×1015 years, but doesn't appear to decay by IT and instead by 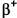 /EC.
/EC.
Shown are three Internal Transition gamma emitters: xenon-127 (also 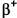 /Electron capture), selenium-77 (also stable), cobalt-60 (also
/Electron capture), selenium-77 (also stable), cobalt-60 (also 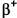 decay) and tantalum-180
decay) and tantalum-180 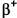 /Electron capture).
/Electron capture).
See 'Internal Transition. and
'Nuclear Spin'.

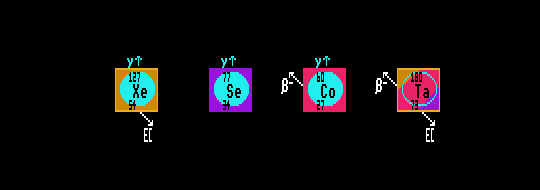
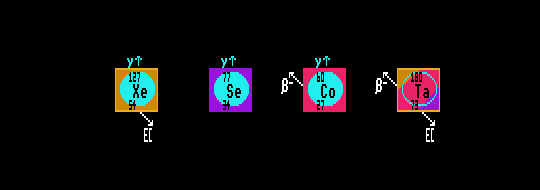
![]() /EC.
/EC.
![]() /Electron capture), selenium-77 (also stable), cobalt-60 (also
/Electron capture), selenium-77 (also stable), cobalt-60 (also ![]() decay) and tantalum-180
decay) and tantalum-180 ![]() /Electron capture).
/Electron capture).
![]()