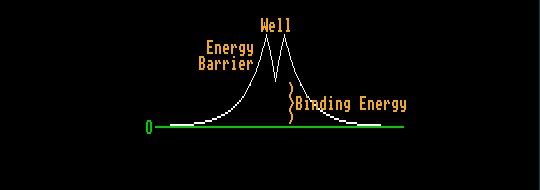

The nucleons within the nucleus do not have sufficient kinetic energy to surmount this energy barrier. However, this large energy could be borrowed if it were borrowed for a sufficiently short time to satisfy Heisenbergs' uncertainty principle. There is a small but finite probability that this energy could be borrowed. The higher the energy barrier, the smaller this probability. This process is known as tunnelling. The alpha particle sometimes finds itself at the same height (energy) but on the opposite side of the energy barrier, having somehow travelled at the speed of light to get there. Containing like positive charges, the alpha particle and daughter nuclei then repel each other, accelerating down the foot slopes of the energy barrier, releasing the difference in energy (binding energy) as kinetic energy. This is alpha decay, and is very much like spontaneous fission, except that instead of the nucleus splitting into two nearly equal parts, it splits up into two very unequal parts.
Alpha decay occurs more readily than spontaneous fission, except for very heavy nuclei (Z>105).
![]()