
|
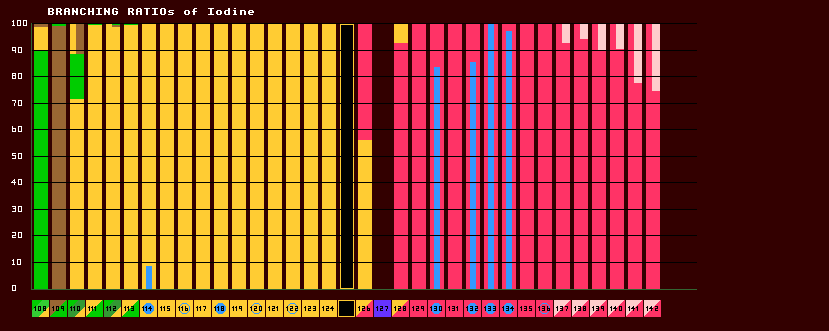
Some unstable isotopes are able to decay by more than one route, called multi-modal decay. The branching ratio is the percentage allocated to each differing route. The sum of all branch routes is necessarily 100%.
Some few isotopes decay by as many as five different routes, most by less than three. In this program, it has only been possible to show the first three most significant decay modes, any not shown are generally neglibible. The branching ratios are displayed as a cumulative histogram, with the most favoured route at the bottom and the least probable at the top. Like the square isotopes below, they are colour coded according to decay products and not decay route. For example: red for both A few of these isotopes have enormously long halflives (drawn in striped blue), and are essentially stable; but they do decay and the decay route still adds up to 100%, so these isotopes are correctly shown with a non-zero branching ratio. Care must be used in interpretion! Metastable states have their own separate branching ratios, shown by the thinner bars in the middle and by the cyan-coloured figures. The metastable decay may involve IT (internal transition) with/without one or more extra decay routes. Only two such metastable decay routes can be shown, metastable (cyan) and fissile (magenta). Some isotopes have metastable states that involve multi-modal decay without any internal transition (IT); it has not been possible to show these at all in the program. The above diagram (for Iodine) is just another (probably highly un-conventional) way of showing the branching ratios of isotopes. But it does show the decay channel proportions at a glance, rather than by studying the numerical percentages. The modal decay path (the path with the greatest percentage) is shown at the bottom of the histogram bars. Another way of showing branching ratios is depicted below. Five examples are shown.
|
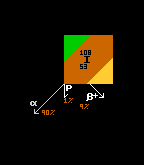
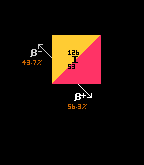
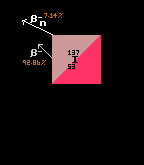
|
Iodine-108 has three decay channels, or modes of decay. The modal pathway for I-108 is by alpha decay (shown in green at 90%). The second most probable decay route (or decay pathway) for I-108 is by inverse beta decay (yellow, at 9%), whilst the least probable (at 1% probability) is by proton decay, where a proton drips out.
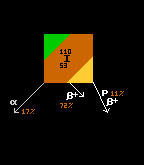
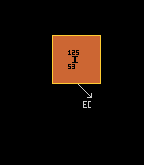
Iodine-110 also has a tri-modal decay path. The most probable decay mode is by inverse beta decay, at a probability of 72% (shown in yellow). The alpha pathway (shown in green) is second most probable at 17%, whilst least probable is the (proton plus positron) pathway at 11% (shown in brown). Iodine-125 has only one decay route, that of electron capture (brown with yellow outline), so is 100% probable. Iodine-126 is one of those isotopes that is both beta and inverse beta unstable, being between two isotopes with higher binding energy (not depicted here). The modal pathway is by inverse beta decay (shown in yellow) at 56.3%, followed closely by the beta decay path (shown in red) at 43.7%. See Even Mass Beta Stability. Finally, iodine-137's modal pathway is by beta decay (red, 92.86%) with the second pathway (beta plus neutron) (shown in pink) at just 7.14% probability. When an isotope is in an excited (meta-stable) state, it has a possibility to decay by two routes. Thus, iodine-130 in it's ground state can decay by only one route (beta decay, red, 100%), but in it's excited metastable higher-energy state, it can decay by either beta decay (red, 18%) or by internal transition (IT) (cyan, 82%). [It has not been possible to show the branching ratios of metastable states in the 'arrow diagrams' like those immediately above]. |
![]()