|
6 CARBON C (Latin: carbo = charcoal)
Found naturally in three allotropic forms, graphite, fullerenes, and transparent white gemstone diamond or cubic diamond (diamond can also exist in the hexagonal diamond crystal structure, first found in meteorites, and thought to be unstable with respect to cubic diamond). Even normal cubic diamond is metastable at ordinary pressures, that is, graphite is just a few electron volts more stable than is diamond, but there is a large energy barrier between the two preventing diamond from reverting to the more stable graphite. Small synthetic industrial diamonds are made by subjecting graphite to immense pressures, but are unsuitable as gemstones. More diamond is now made synthetically than mined. Diamond films can be grown in the laboratory by vacuum deposition.
|
Diamonds
Synthetic gemstone quality diamonds of up to 1 carat can now be produced by mixing graphite with troilite, an non-magnetic iron sulphide of composition FeS found in meteorites, and subjecting it to immense pressures in a press with a seed diamond. By careful control of the temperature gradient, cooler towards the seed, the atoms of carbon dissolve in the troilite and migrate towards the seed diamond which grows. A powerful magnetic field pulling away from the seed is probably used to prevent iron inclusions from forming within the growing crystal of diamond, as otherwise a magnetic diamond is indicative of a synthetic diamond! To prevent atomic nitrogen impurities imparting a yellow-brown colour, a nitrogen-getter like aluminium is used. Natural diamonds still contain nitrogen, but are colourless because the diamonds were liquid long enough for the lone atoms of nitrogen to group together to form pairs or quadruplets. However, the artificial diamonds are still distinguishable from natural diamonds by their bluish fluorescence in intense short ultraviolet light, and subsequent 5 second phosphorescence for after its removal, but scientists are working on this. Some natural diamonds also fluoresce, but do not phosphoresce.
Diamonds one and a half times harder than any before measured, either natural or artificial, have now (2004) been produced in a two-stage process. A plasma of a mixture of hydrogen and methane deposits carbon onto a pre-existing thin diamond substrate, forming a thick film of diamond, which is then subjected to temperatures of 2000 Celsius and pressures of 70,000 atmospheres, producing diamonds up to 5mm thick and with exceptional hardness. deBeers, the largest diamond merchants in the World, are very worried; they keep the price of natural diamonds artificially high by restricting the selling of the masses of diamonds they have already mined. What a shame.
Natural diamonds are formed deep within the Earths crust in the oldest parts of continents, known as cratons. Here the rocks have not changed since the Archaen period, 2.5 billion years ago. The outer shell of the Earth is thick here, and the pressure great enough for diamonds to form. It is thought the carbon from which the diamonds are made started as carbon-rich sediments on the ocean floor that were subsequently subducted down with the basalt to depths of hundreds of kilometres where the pressure is very high but the temperature relatively low for by upper mantle standards. The evidence for this is that diamond bearing kimberlites contain eclogite, a rock formed from subducted basalt. But few diamonds ever make it up to the surface, because as they rise, the pressure on them decreases and the crystal structure reverts to graphite unless they are eject at depth with great speed. Gas-rich magma would provide such conditions, with the gas being released explosively, blasting the diamonds up a tapering hole, or pipe, around which forms a volcanic rock called 'kimberlite'. Finding a kimberlite pipe almost guarantees that diamonds are present near the surface. Garnet found in stream-beds are also associated with diamonds from kimberlite pipes, find one, and the other may well be present nearby.
Simulations have shown that diamond, subjected to temperatures exceeding 6000 Kelvin and pressure upwards of a million atmospheres (too extreme to generate in the laboratory) can exist as a liquid that will flow. Diamonds may have been formed from carbon early in Earths history this way, provided that the liquid diamond formed was then cooled rapidly.
Diamond is based on an adamantane structure, a three dimensional network of interconnecting hexagons, with each carbon atom bonding in a tetrahedral arrangement to four nearest neighbours. Thus formed, the cubic lattice of diamond is immensely strong, but relatively empty.
Diamond is transparent and one of the hardest minerals known, used at the tips of metal-cutting tools, and as an abrasive. Its high refractive index of 2.4 makes diamond gems sparkle. It can be manufactured artificially at high temperatures and pressures, and is believed to comprise part of inter-stellar dust especially around hot carbon-burning stars such as red-giants. Diamonds burn in air at 700 Celsius forming carbon dioxide, CO2. Diamond conducts heat better than graphite (and 20 times better than copper), and is used to conduct heat away from specialised electronic devices. Diamond dust can be formed by progressively heating soot in a laser beam to 2800ºC, the diamonds so produced being free of nickel and nitrogen impurities that colour synthetic diamonds yellow, this process also produces traces of two high-pressure forms of carbon, chaoite and lonsdaleite. Diamond can now be grown as a coating on other materials by CVD (chemical vapour deposition), where the part to be coated is put in a chamber filled with hydrogen and a hydrocarbon gas at reduced pressure at a temperature of xxx Celsius. The diamond film gradually grows at a rate of 1 micrometre per hour on the cooler part. The Mistry process is said to deposit diamond films 4000 times faster: the object to be coated is held in an atmosphere of carbon dioxide and nitrogen gas and blasted by a laser which heats a spot on the surface to 10,000 Celsius where the diamond forms by an unknown mechanism. The low friction and hardness of diamond coatings make it useful for coating gear teeth and surgical blades etc. Diamonds are hydrophobic but will stick to grease. Diamonds fluoresce with a blue light under X-ray bombardment, some under ultraviolet light, which provides a test for diamond. Diamond is one of the few materials known with a negative electron affinity.
|
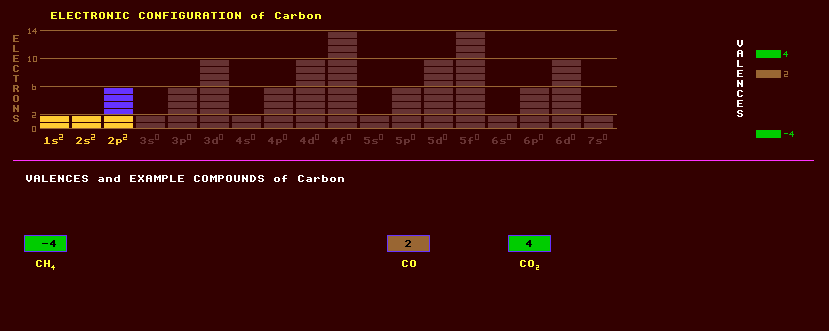
Carbon is tetravalent, with it's bonds being directed to the apex of a regular tetrahedron. The basis of all life and organic chemistry. Alloyed in small amounts with iron, it forms steel; in larger amounts, cast iron.
Graphite consists of layers of a mesh of carbon atoms joined in hexagons, rather like chicken wire mesh, with the layers stacked alternately above each other on two different centres. This is hexagonal graphite. Graphite with a rhombohedral crystal structure is also known naturally, where the layers are stacked upon each other on three different centres. Natural graphite usually consists of a mixture of 80% hexagonal graphite, 14% rhombohedral graphite, and 6% disordered.
Graphite is shiny black, feels greasy, conducts electricity and is a near black-body: a good radiator of heat. Graphite is used as electrodes in Zinc Carbon Lechlanche cells and in carbon-arc cinema projector light sources; as commutator brushes in electric motors; as the resistive element in resistors; as the 'lead' in pencils (so called because 'plumbago' was the old name for graphite); as a dry lubricant; and as the electrically resistive element in carbon resistors and volume controls. Old telephone microphones used to be made with carbon granules as the sound sensing element. Colloidal graphite is used as a coating on valve anodes to inhibit the production of secondary- and photo-electrons in CRTs. Used as a moderator of neutrons in nuclear reactors. Graphite conducts heat better in some directions than others, and five times better than copper. Due to its very high melting point, once used as a filament in evacuated incandescent lamps, now superseded by tungsten. Carbon fibres 8 micrometres in diameter greatly strengthen composite materials, e.g. fishing rods. Graphite occurs naturally by the metamorphism of coal, but may also be of inorganic origin. Shungite, or lump graphite, is a coal-like rock mined in Russia.
Graphene is a single one-atom-wide sheet of graphite, once thought to be un-stable, but now found in pencil strokes on paper. Sheets containing up to a million carbon atoms have been identified. If an electrical current is passed across in one direction, it will modulate a weaker current trying to be passed in another direction, and could thus be utilised as a very thin transistor.
NFC or near frictionless carbon is a new way of evaporating an amorphous carbon film by way of an rf field onto any substrate. It is ultra-hard with a coefficient of friction of 0.001, being twenty times lower than that of the previous record holder molybdenum disulphide. It has exceptional wear resistance and has promising uses in turbo-chargers and space gyroscopes, etc.
A metastable cubic form of carbon with 24 atoms per unit cell was formed by subjecting graphite to a pressure of 150kbar. Yet other forms are suspected.
|
Fullerenes
A third allotropic form of carbon called Buckminster-Fullerene shaped in the form of a football or truncated icosahedron, formula C60, which has pentagonal symmetry, forms spontaneously in 10ppm quantities in the soot from a carbon arc or candle flame and will dissolve in benzene, toluene and hexane forming magenta crystals that crystallize in the body-centred cubic lattice. The sooty burning of benzene or acetylene produces up to 0.3% C60 but the yield can be increased to 20% by putting a carbon arc in an inert gas atmosphere heated to 1300 Celsius. Fullerenes have been found in shungite (see above) and in fulgurite, a glassy dendritic mass formed when lightning strikes the ground. Fullerenes decompose slowly in air and exposure to light, but might be stabilised by forming compounds. The electrons in C60 form a closed shell, which makes it more stable, and every carbon atom has identical surroundings. The spheres of fullerenes in crystals rotate at 20×109 times per second. Smaller amounts of deep red rugby ball shaped C70 are also produced in smoky flames; it too has a closed electronic shell but the 70 carbon atoms find themselves in 5 different environments.
A whole zoo of different fullerenes with closed and nearly spherical shells of interconnected hexagons and pentagons is now known starting from C28 and increasing in increments of always two carbon atoms to C84 and well beyond. C76 is bright green, C78 golden yellow, and C84 olive green. All fullerenes have an even number of carbon atoms, and must have 12 pentagons in order to be closed 'spherical' structures.
Some fullerenes are more stable than others: thus C28 is the first to avoid a fused quartet of pentagons, C50 is the first to avoid a fused triplet of pentagons, C60 the first to have completely isolated pentagons and C70 the next to have completely isolated pentagons. C76 is the first chiral fullerene existing in two mirror image forms, C78 the first for which there are more than one isomer (there are three, one pair are enantiomorphic or chiral). Chirality, or handedness, is common for the higher fullerenes. Beyond C100 they are no longer soluble in benzene. C240 and C540 are symmetrical fullerenes. As the cages get bigger on the larger fullerenes, the shape becomes less spherical with the 12 pentagons become sharper and sharper corners until they take on the shape of an icosahedron.
Nesting concentric spheres of fullerenes (hyperfullerenes) and nesting cylindrical forms (Bucky tubes) are also easy to make in carbon arc discharges, as is buckminster fullerene itself. But the yield of C50 is very low because of its highly strained structure, however, the yield can be enormously increased by adding chlorine gas to the vaporised carbon produced by the electric arc. Here a ring of 10 atoms of chlorine girdles the forming structure like a tight belt reducing the formation of C60 and increasing the production of C50 instead.
Up to 6 metal atoms can be accommodated in the niches between the regularly spaced crystallized fullerene spheres to form fullerene compounds. Alkali metal fullerene compounds are superconducting, K3C60 has a superconducting transition temperature of 19.2 Kelvin and RbCs2C60 one of 33 Kelvin, but Cs3C60 holds the fullerene record at 40 Kelvin. Alkaline earth metal superconducting fullerenes are also known, Ca5C60 (8.4K) and Ba6C60 (7k). Fullerenes can also trap atoms within the spheres themselves, forming clathrates such as La@C60. Other endohedral compounds have been made with atoms of titanium, scandium, caesium, potassium, uranium and the lanthanides within, but not with aluminium, iron, nickel, copper, silver or gold which seem to shun fullerenes. Up to four metal atoms have been so trapped within a fullerene cage. C28 has four bonds arranged tetrahedrally like carbon itself and it may be possible to form another new diamondic allotrope of carbon with C28 behaving as would single carbon atoms.
A uranium atom will sit inside C28 bonding with the four bonds and stabilising it. Fullerene cages can be fused producing larger multi-caged structures. Buckminster fullerene is thought to be produced in vast quantities in the outpourings of carbon soot from red giant stars. He@C60 has been found on Earth associated with a meteorite impact crater from 1.85 thousand million years ago in Ontario, the fullerene must have been formed near a red giant star because the helium-3 to helium-4 ratio of the trapped helium is of a quite different ratio to that found on Earth. Buckminster fullerene has also been found (with the anomalous iridium) at the K/T (Cretaceous/Tertiary) boundary layer in rocks marking a major dinosaur extinction event that happened only 65 million years ago when it is presumed a large meteorite having an excess of iridium crashed down to Earth in the Yucatan peninsula, Mexico. See Iridium.
|
Aliphatic hydrocarbons are compounds of carbon with hydrogen bound in a chain (which can be branched). Carbon can form single bonds as in ethane H3C-CH3, double bonds as in ethylene H2C=CH2, or triple bonds as in acetylene HC CH which release a lot of energy when broken e.g. in oxy-acetylene welding. Aromatic hydrocarbons are carbon ring compounds based on benzene, C6H6. Interestingly, some hydrocarbons based on geometric solids including four of the five Platonic solids: tetrahedrane, C4H4, octahedrane (isomeric with benzene), C6H6, cubane, C8H8, and dodecane, C20H20 have been synthesised along with a triangular prism shaped molecule and explosive liquid called prismane, C6H6, another isomer of benzene. Other ringed isomers of benzene include fulvene, C5H4=CH2, Dewar benzene consisting of two fused square rings, and benzvalene a caged ring molecule with foul odour. Adamantane, C10H16, consists of four fused benzene rings forming a diamond lattice like cage structure. Many tens of thousands of other carbon compounds are known, half of all chemistry is based on the compounds of carbon. CH which release a lot of energy when broken e.g. in oxy-acetylene welding. Aromatic hydrocarbons are carbon ring compounds based on benzene, C6H6. Interestingly, some hydrocarbons based on geometric solids including four of the five Platonic solids: tetrahedrane, C4H4, octahedrane (isomeric with benzene), C6H6, cubane, C8H8, and dodecane, C20H20 have been synthesised along with a triangular prism shaped molecule and explosive liquid called prismane, C6H6, another isomer of benzene. Other ringed isomers of benzene include fulvene, C5H4=CH2, Dewar benzene consisting of two fused square rings, and benzvalene a caged ring molecule with foul odour. Adamantane, C10H16, consists of four fused benzene rings forming a diamond lattice like cage structure. Many tens of thousands of other carbon compounds are known, half of all chemistry is based on the compounds of carbon.
Carbon forms compounds with almost every other element in the periodic table, perhaps the only exceptions being with the noble gases. Three oxides of carbon are known: carbon monoxide, CO, an inflammable, odourless and poisonous gas; carbon dioxide, CO2, an odourless gas; and carbon suboxide, C3O2, O=C=C=C=O. Higher oxides in this series are known, O=C=C=C=C=C=O being another. Since carbon dioxide has an atmospheric residence time of only 3 years, the concentration of atmospheric CO2 can vary quite quickly, and has been increasing from an initial 250 ppm over the last 30 years, due largely to destruction of tropical forests which 'breath in' CO2 during photosynthesis, and the burning of said forests, which emit CO2. The concentration of atmospheric carbon dioxide is now (1997) at about 360ppm and is increasing by 0.4% of this value yearly. Because carbon dioxide is a greenhouse gas and contributes to global warming this represents a considerable threat to life on Earth through climatic changes. Apart from water vapour, the greatest contribution to global warming is made by the gaseous compounds of carbon. The contribution of carbon dioxide to the greenhouse effect is 61%, atmospheric methane 16%, the CFC's a further 12%, and carbon monoxide 1%. The oxides of nitrogen contribute the remaining 10%.
Carbon consists of two stable isotopes, 99% carbon-12 to which atomic masses are referenced, and 1% carbon-13. Carbon-14 is continuously created in the Earths atmosphere by the absorption of cosmic ray neutrons by nitrogen-14 nuclei and is the basis of radiocarbon dating. Carbon-14, a beta-emitter with a halflife of 5730 years, is absorbed (along with carbon-12) by living organisms. After death, the ratio of carbon-14 to carbon-12 decays exponentially with time and the year of death can be calculated. Carbon-14 is also produced in the atmosphere by atmospheric nuclear explosions, which would be a significant worldwide hazard after a nuclear war.
Diamonds occur naturally in pipes of kimberlite, a greeny grey-bluish rock formed from altered peridotite which is from the Earths mantle. Both occur deep underground at a depth of 100 - 200km where temperatures are about 1200 Celsius. Diamonds often occur as mis-shapen octahedrons. Diamond cleaves easily in four planes, corresponding to the sides of an octahedron. Only 23% of natural diamonds are suitable for use as precious gems, the rest are used as industrial diamonds. The largest unblemished specimens are faceted in the 'brilliant cut', the smaller less valuable in the 'rosette cut'. Blue diamonds contain minute traces of boron. Graphite forms naturally in metamorphic rocks including slate and schists. Charcoal, a porous form of carbon, is obtained by burning timber whilst excluding most air. Activated charcoal was used to absorb noxious gases within WWII gas masks and is still used as filters. The vast majority of carbon on Earth, 99.9% of it, is present in rocks, mostly carbonate rocks.
Claim to fame: As diamond, it has the highest atomic concentration (18x1022 atoms/cc); the lowest compressibility (1.8x10-12m2/N); the highest bulk modulus; the highest melting point (3823K); the highest thermal conductivity (23W/cmºK); the lowest linear temp. coeff. of expansion (1.2ppm/ºC); the lowest specific heat (6J/ºK/mol); the highest Debye temperature (2230 Kelvin); the lowest (solid) entropy (2.3 J-1 K mol-1); the hardest - 10 on Mohs scale of hardness; and has the highest latent heat of fusion (105KJ/mol) of any element (and quite possibly of any other compounds!).
|

![]()