
|
77 IRIDIUM Ir (Latin: iris = rainbow)
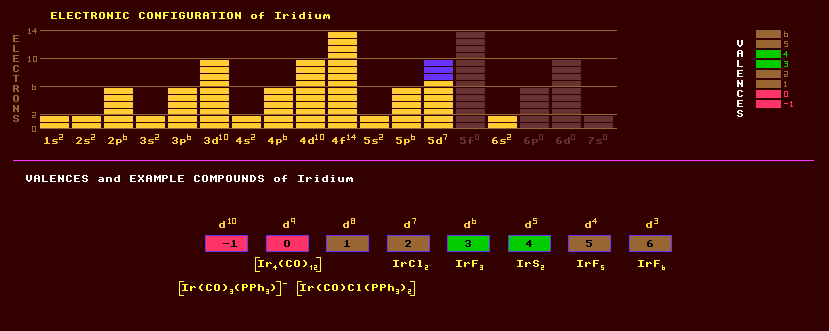
Iridium is a hard, brittle, lustrous silvery metal belonging to the platinum group. It is unaffected by air, water and all acids, but fused caustic soda will attack it. Iridium is used in special alloys and in spark plugs. Alloyed with platinum or osmium it forms hard corrosion resistant alloys used for pen nibs, watch and compass bearings, laboratory crucibles and standards of length. The standard metre in Paris consists of an alloy of 10% iridium and 90% platinum. Iridium occurs naturally in the alloy osmiridium OsIr which can contain small amounts of platinum, ruthenium and rhodium; and as iridosmine, [Os,Ir] (with less than 65% iridium) which crystallizes in the hexagonal system. It occurs in alluvial sands. Iridium is also found in platinum ores or in compounds with tellurium. Iridium is one of the rarest elements in the Earths' crust, but is more abundant in some metallic meteorites and probably within the Earths' core. There is a thin layer of Buckminster Fullerene-containing soot and ash containing excess iridium, the iridium anomaly, scattered uniformly over the Earths surface at Cretaceous/Tertiary boundary (K/T boundary) of 65 million years ago. It is theorised that a large meteor containing excess iridium crashed into the Earth 65 Million years ago darkening the skys with ash and killing off the dinosaurs. There is a arge crater under the sea at the Yucatan Peninsula, Mexico, dating back 65 million years. The date is highly significant: it has been calculated that when Jupiter and Saturn line up precisely with the Sun and one (or more) asteroids finds itself in a resonance orbit with Jupiter, this can swing the solar system into a chaotic state, where Saturn and Jupiter can alter course or orientation, and the asteroid be flung into a very different orbit, some crossing Earths orbit. Precision calculations have revealed that the solar system went through a chaotic phase 65 million years ago, and this may have swung an asteroid on an Earth collision orbit. Another chaotic phase is predicted in 30 million years hence, and possibly one 250 million years ago at the end of the Permian and the beginning of the Triassic period (although the calculations accumulate larger errors over this timescale), 251 million years ago, when there was another mass extinction event on Earth. Iridium exhibits a range of valences from -1 to 6 inclusive, but valences of 3 and 4 are preferred. Example compounds include [Ir(CO)3(PPh3)]-, Ir4(CO)12, [Ir(CO)Cl(PPh3)2], IrCl2, IrF3, IrO2, IrF5 and IrF6. Iridium is named after a rainbow because its salts are highly coloured. Osmium and iridium are the two densest elements known. The high density of osmium and iridium is due to the poor shielding of the high positive nuclear charge by the f-orbital electrons which are slowly filled in the lanthanide series. The f-orbitals have many lobes with large gaps allowing the nucleus to pull the outer electrons of the transition metal elements that follow them towards the nucleus, thus making them small, and endowing upon them high density. This also accounts for their high ionization energies (esp. mercury) and low chemical reactivity (esp. gold and platinum). Measurements suggest that osmium is slightly more dense than iridium, but calculations suggest the other way round, with iridium being 22.65 and osmium only 22.61. Interestingly, the crystal structure of osmium is hexagonal close packed whereas that of iridium is the more loosely packed face centred cubic. Naturally, iridium consists of two stable isotopes, comprising 63% iridium-193 and 37% iridium-191. A further 31 radioactive isotopes are known, ranging from the alpha decaying iridium-166 to the beta decaying iridium-198. The radioactive iridium-192, a beta decaying isotope with a halflife of 74days, is used as a medium energy gamma ray emitter in industrial radiography. Claim to fame: Iridium or osmium is the most dense element, with a density of 22.6 grams per cubic centimetre.
|
![]()