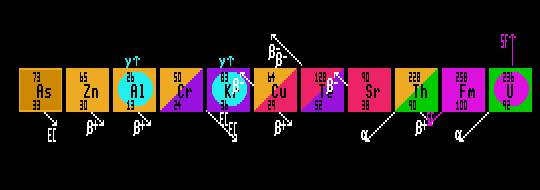
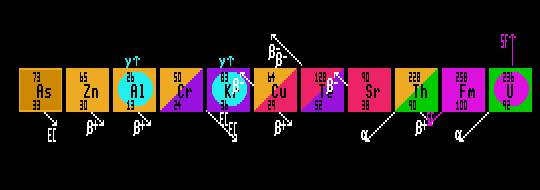
Some nuclides have such enormously long half-lives (5.5×1024 years) compared to the present age of the Universe (approx. 13.81×109 years) that they may be classed as stable but slightly radioactive. When a nuclide decays, it may transmute into a different element of lower or identical Atomic Weight (A).
Elements with Atomic Number, Z, greater than 92 (uranium) are only produced in high energy sub-atomic particle accelerators, nuclear reactors, super novae, or nuclear explosions; as they are all unstable. There are no stable isotopes for Z=43 (technetium), or any element above and including Z=84 (polonium). Similarly, there are no stable isotones for N=19, 21, 35, 39, 45, 61, 71, 89, 115, and 123, all odd numbers. Also, A=5 and A=8 are the only isobars below A=211 for which no stable isobars exist.
Shown: arsenic-73 (electron capture), zinc-65 (inverse beta decay), aluminium-26 (isomer & inverse beta decay), chromium-50 (Dbl Inverse Beta), krypton-89 (otherwise stable isomer [Internal Transition gamma emitter]), copper-64 (dual-mode beta/inverse beta), tellurium-128 (Dbl beta decay), strontium-90 (beta decay), thorium-228 (alpha decay), fermium-258 (Spontaneous Fission) and uranium-236 (alpha decay & fertile isomer [SF]).
![]()