The transmutation of Iodine-129 into Iodine-128

But it has been achieved by the use of an extremely powerful laser, of a type so powerful that construction has only recently been possible. The laser in question is called 'Vulcan' and is as big as a small hotel and located at the Rutherford Appleton Laboratory in Oxfordshire. It is, in 2003, the most powerful laser in the World.
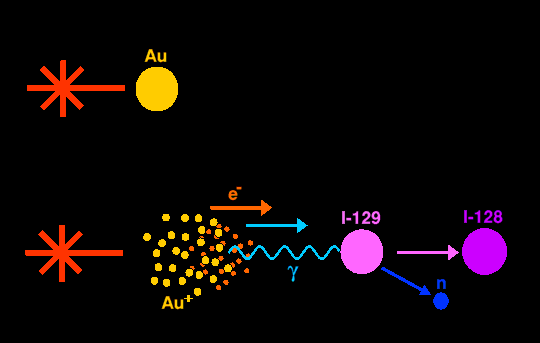
Vulcan can be used to add protons to the nuclei of lighter elements. Alchemists used to dream of turning mercury into gold. The reverse has been accomplished, turning gold into mercury. The same laser has also changed oxygen into fluorine, and iodine-129 into iodine-128.
Iodine-129 has a half-life of 15.7×106 years, whilst that of iodine-128 is a mere 25 minutes. Thus the technique holds promise for transmuting the highly radioactive waste of nuclear power stations (and the like) which have long halflives into isotopes with much shorter half-lives (but with much higher short-term activity), thereby eliminating the long-term disposal hazard.
To accomplish the conversion of iodine-129 into iodine-128, the laser fired an intense pico-second pulse at a heavy metal target, gold in this case, vaporising the gold atoms into a plasma of electrons and nuclei, which then emit gamma rays as they pass through the rest of the target. The intense burst of gamma rays then collided with the atoms of iodine-129 shaking the nucleus of iodine-129 so hard that a neutron is shook out, leaving iodine-128. But this process is enormously in-efficient because the light has first to be converted into gamma rays, and then only a small fraction of these gamma rays interact with the true target. The experiment converted only 3 million atoms of iodine-129 into iodine-128, less that femto-gram. To convert the whole test sample the laser would have to have been fired 1017 times, but it is currently only capable of being fired once every hour. Clearly this is a very in-efficient process for which a whole nuclear power station would have to be dedicated, thus generating more radioactive waste than the laser can possibly destroy!
Obviously, destroying the vast quantities of nuclear waste is not an option for the present laser, but making minute quantities of highly radioactive (short half-life) isotopes used in medical imaging may be practicable. Fluorine-18 decays by inverse beta decay, emitting a particle of anti-matter, a positron. When fluorine-18, given as an injection, decays within the human body, the positron it emits on decaying is likely to encounter an electron belonging to another atom, and when it does, both will mutually anihilate each other, leaving two tell-tale 500MeV gamma rays, which are emitted at the point of annihilation. By detecting these gamma rays the internals of the human body can be imaged. The technique is called positron emission tomography, or PET for short. For this, inverse beta decaying isotopes are needed, and they must have a short half-life in order that there is a good chance that they will decay whilst the body is being imaged. If an isotope with a longer half-life is used, then more of it will be required, and it will continue decaying long after the body scan is complete, un-necessarily irradiating the subject. Fluorine-18 has a halflife of 1.83 hours thus satisfying the short half-life criterion. But because it decays so fast, it must be created on demand just before the time of use. Inverse Beta decaying isotopes are not present on Earth, and must be created artificially. Presently, these isotopes are made using particle accelerators, which need heavy shielding. They are very expensive, and very few hospitals can afford them. This is where smaller table-top versions of the laser come in, which may be available in the year 2008.
![]()
![]()