
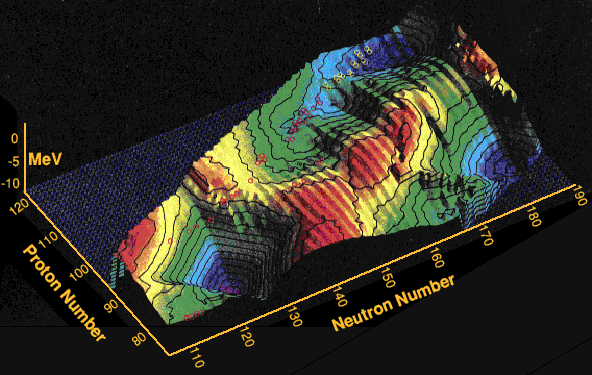
The above diagram shows the calculated shell-correction energies for super-heavy nuclides. The landscape shows the largest energies in blue, those with the strongest shell-stabilising effects, whilst the red areas show those nuclides with the weakest. Thus the mountain ranges are least stable, whilst the valleys have greater stability. The red circles show newly discovered nuclei, the yellow are unknown as yet but thought to be manufacturable from fusion reactions with lead-208 targets. The nucleus with 174 neutrons and 116 protons is right in the middle of the deep blue lagoon of relative stability at the top right of the diagram. Note that none will actually be stable, all will be radioactive, but the ones in the blue lagoons should have longer halflives than those surrounding them. The lagoon at the bottom right is thought to be in-accessible with present heavy nuclei production methods.
Previously, production of elements 101 to 106 was achieved by bombarding actinide targets like curium, californium or einsteinium with helium, carbon, oxygen or neon ion projectiles. But these targets are expensive and only available in milligram quantities. Moreover, the binding energies of the targets is fairly low, and any fusion between target and projectile leaves the resulting nucleus with a large excess energy and liable to either shed 3 or 4 neutrons or split asunder immediately, decimating the production rate.
But by using near-magic stable elements with much greater binding energies as both targets (lead or bismuth) and projectiles (calcium to nickel), the resulting fused compound nucleus needs shed but one neutron to attain the ground state. Even so, production rates are extremely small requiring about 1018 projectiles to make one atom. It was found that elements 107 to 112 were much more stable than supposed, preferring to decay by alpha decay than by the (much faster) spontaneous fission route. The stability increases around N=162 and Z=108 because this nucleus is a prolate spheroid. Superheavy element 116 (with N=174) might be made by bombarding lead-208 with selenium-82.
A typical experiment producing a super-heavy element is herewith described: One atom of element 112 was created on the 9th February 1996 by the team at the GSI heavy ion research centre at Darmstadt in Germany. The atom of element 112 had a mass of 277 and was prepared by bombarding a target of lead-208 with 5×1018 ions of zinc-70 in the tenth ionization state at speeds of 30,000 kilometres per second and a kinetic energy of 343.8MeV. The cross section for this reaction was measured as 1picobarn.
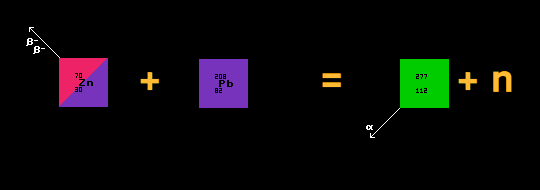
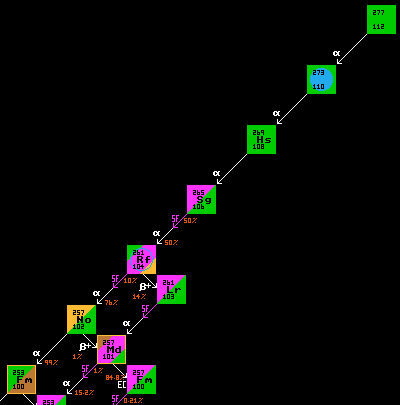
All transfermium nuclei yet produced are far left of the calculated beta/inverse-beta watershed which traverses point Z=114, N=180. They are thus unstable to inverse-beta/EC decay. Most are also unstable to alpha decay.
![]()
![]()