S-PROCESS NEUTRON CAPTURE
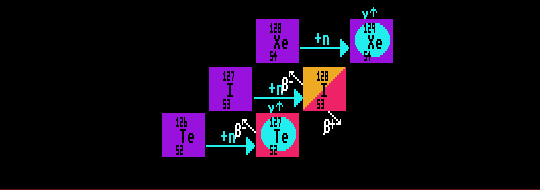
In the s-process (slow process), there is sufficient time for newly created radioactive nuclides to decay before the next neutron capture event occurs. This can only happen when the neutron flux is low. The decay path can be by alpha decay or beta decay. Thus the stable tellurium-126 will absorb a neutron to become the beta-unstable tellurium-127, which has enough time to decay into iodine-127 before it can capture another neutron to become tellurium-128. The iodine-127 so produced is stable and absorbs another neutron to become iodine-128, which decays by beta decay into stable xenon-128 before the iodine-128 can capture another neutron to become iodine-129. The stable xenon-128 so formed captures another neutron to become stable xenon-129. The process continues through more stable xenon isotopes until it produces xenon-133, which is unstable, and decays into caesium-133, which absorbs another neutron... and so on. The production of certain isotopes (tellurium-128 in this instance) is thus blocked in the s-process by the decay of any unstable intermediates.
The s-process, build up of radionuclides occurs along a route which keeps close to the line of stability, but cannot proceed beyond those whose atomic mass exceeds 209, where alpha decay becomes fast. The natural occurrence of nuclides with atomic mass above 209 means that they were produced in the r-process.
The s-process is thought to occur within red giant stars where there are neutrons, but not in sufficient numbers for the r-process to occur. Some nuclides can only be produced by the s-process, whilst others by only the r-process. Many are produced by both processes.
From any chosen seed, the nuclides produced by the s-process can be displayed by depressing the [S] key when displaying any Segre chart. The stable end products of the s-process are highlighted by a white square, whereas the temporary radioactive beta decaying products are highlighted by one of cyan.



![]()