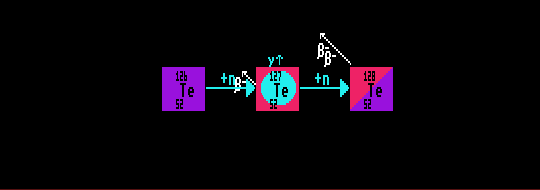

The r-process build up of radionuclides occurs along a route which takes it far from the line of stability right up to the neutron drip line, where no further neutron capture can take place. Further build up is prohibited unless a quick beta decay process can occur. The r-process can only occur when the neutron flux is extremely high, such as near supernovae or other cosmic catastrophes. After the r-process has finished, the highly unstable nuclides will decay by beta decay into more stable nuclides with longer halflives, but remaining on the neutron rich side of the stability curve.
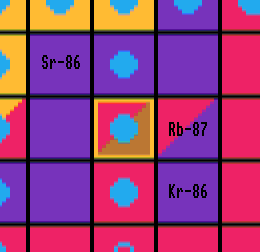
Some nuclides can only be produced by the s-process like strontium-86 and strontium-87 which are shielded from the r-process by krypton-86 and rubidium-87 respectively because these are either stable or have long half-lives. Se diagram below.
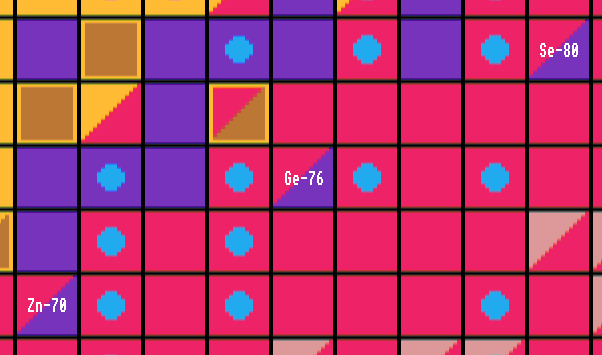
Others like zinc-70, germanium-76 and selenium-80 by only the r-process because they have extremely long half-lives. Many can be produced by both processes. The r-process is the main way in which elements heavier than bismuth were synthesised. Diagram below.
![]()
![]()