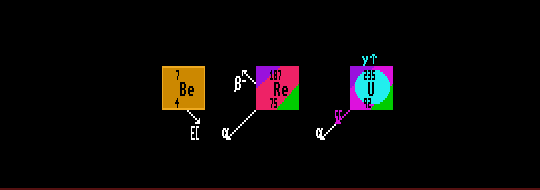
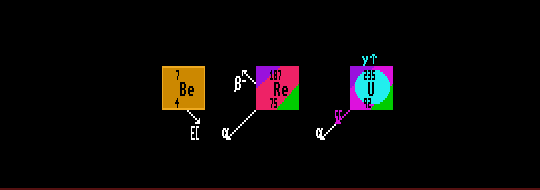
The largest changes in halflife are expected for those nuclides subject to electron capture, as these involve the capture of an electron from an innermost electron shell by the nucleus. Beryllium, having its four electrons close to the nucleus, exhibits the largest effect. Beryllium-7 decays by electron capture to lithium-7 with a halflife of 54 days. Changes in the electron density occurring in different compounds of Be-7 have been observed to affect the halflife of it by up to 0.18%. An increase in pressure of 270Kbar on beryllium oxide, BeO, resulted in a change in halflife of 0.6%.
Theoretically, smaller changes in the halflives of beta emitters should be possible as alterations in the electron cloud would affect the force felt by the emerging electron in beta decay. An extreme case is that of rhenium-187, a beta emitter which decays to osmium-187 with a halflife of 43.5×109 years. Re-187 has a beta decay energy of the incredibly small value, by nuclear decay standards, of only 2.6keV, which is why it has such a long half-life. When rhenium-187 is fully ionised, it should, theoretically, be stable, as the decay energy only has to change by more than 2.6keV for this to be possible. See Beta Deay Enhancement.
Changes in the alpha decay rate of samarium-147 by chemical effects are calculated to be less that 0.000015%. Those of radium-226 ten times less. Other alpha emitters have changes of the same order, i.e. 1 part in 107 or less, which is an immeasurable difference, practically, since most halflives are known, at best, to only three decimal places.
Changes in the halflives of excited nuclides or nuclear isomers by internal conversion (isomeric transition - which do not involve transmutation) exhibit the greatest effect; a shift in IT halflife of 5.7% has been observed in uranium-235m.
But it has proved possible, at least in the case of some metastable isomers, to massively alter the halflife by irradiating them with other radiation, stimulating the nucleus to decay, creating in effect the nuclear equivalent of lasing. See Gamma Ray Lasers.
It has been shown experimentally that the rate of some fusion reactions is dependant upon the temperature. For instance, when deuterons are fired at a sliver of deteron-soaked tantalum, the deuterons fuse more easily.
|
Rolfs metal-embedded halflife preditions.
Claus Rolfs has found that the rate at which Lutetium-176 fuses with protons is higher if the lutetium is within a metal (rather than within an insulator). Rolfs beleives that the rate at which radioactive beta decay and inverse beta decay is dependant upon whether the element is inside a metal. He reasons that in inverse beta decay, where a proton is converted into a neutron and a positron is emitted, that the electrons within the metal attract the positron out, and will thus shorten the half-life. Conversely, he reasons that the opposite is true for a beta-decaying isotope which emits an electron: the electron is repelled by the electrons in the metal and the half-life will be correspondingly longer. He reckons that when beryllium-7, which decays by electron capture, is embedded within an alloy of palladium and indium at a temperature of only 12K, then the normal half-life of 53 days is increased by 0.8%. Similar experiments with sodium-22, an inverse beta decayer with a half-life of 2.6 years, showed that the halflife decreased by about 1.2% when it was within the alloy at a temperature of 12K. In a further experiment, bombarding the stable gold-197 with neutrons creating the beta-decaying gold-198, which normally has a half-life of 2.7 days, the surrounding metallic gold caused the half-life to increase by a staggering 5 hours, or 7.7%. He predicts that, for alpha decaying isotopes, entombing the alpha decayers within metal and cooling it down to 4K, enormous changes in halflife for some isotopes. For instance, for the alpha decaying radium-226, he thinks that it might be possible to decrease the halflife 1000-fold! For polonium-210 he predicts a 10.6 fold reduction under similar conditions. Others are more skeptical claiming that if such drastic reductions in half-life were possible, they would have been spotted in other experiments by now. Indeed, in a different experiment before Rolfs proclaimed such startling claims, radium-224 has been embedded in iron and chilled to 1K without anyone noticing any change in halflife. Rolfs himself has embedded polonium-210 within copper at a temperature of 14K, but he noticed only about a 10% reduction in half-life, a far cry from his calculated 95% reduction!
|
![]()
![]()