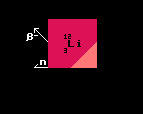 |
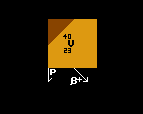 |
 Unstable to neutron decay |
 Unstable to proton decay |
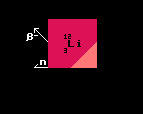 |
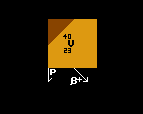 |
 Unstable to neutron decay |
 Unstable to proton decay |
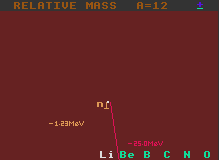
The neutron decay energy is represented as a pink vertical line decending from the central nuclide. Note that, after having decayed, the neutron number decreases by one unit as does the atomic weight, so it does not have the same mass number as the other nuclides represented (the proton number stays constant, so to indicate this, the line is vertical and above the central parent element). Fig.1 shows lithium-12 being unstable to neutron decay with an energy surplus of 1.22MeV. It would decay into lithium-11. It is also unstable to beta decay.
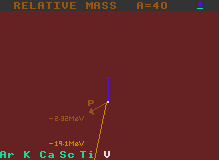
The proton decay energy is similarly indicated by a diagonal sepia line, but in this case after having decayed, the proton number decreases by one unit (as does the atomic weight), so the line ends up above the next lower element. Fig.2 shows vanadium-40 being un-stable to proton decay with a surplus of 2.31 MeV. It would decay into titanium-39. It is also unstable to inverse beta decay.
It is important to stress that, for proton, neutron (and for alpha decay), the resulting daughter nuclide does not have the same atomic weight as for the other decays shown on the same chart (beta, inverse beta, double beta and double inverse beta). But all decays are shown on the same energy scale with the length of the line being proportional to the difference in atomic mass between the parent nucleus and the sum of the masses of the daughter product plus the product particle.

![]()

Note that in !Nuclides, the Proton and Neutron instability energies are only displayed in the 'L H S STAB' mode, not the 'DECAY' mode.
![]()
![]()