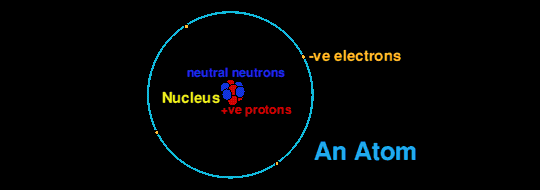

Stable nuclei tend to have about an equal number of protons and neutrons, except for elements heavier than calcium, the twentieth element, which have increasingly more neutrons than protons.
The nucleus is almost incompressible, so the density of the nucleus is roughly constant for light and heavy elements alike. The radius of the nucleus varies from about 1.1fm to 1.8fm from light to heavy elements. The resulting density of the nucleus is extremely high at approximately 2×1014g/cm3, corresponding to a population density of approx 1.4×1038 nucleons/cm3.
A nuclide is distinguished from other nuclides by the number of both protons and neutrons within the nucleus. Isotopes, on the other hand, are nuclides belonging to the same element where only the number of neutrons differs. The radioactive isotopes are called radioisotopes, the radioactive nuclides are called radionuclides. Thus the Segre chart contains at least 3000 different nuclides, both stable and radioactive, but the element xenon (say) has only about 35 isotopes.
![]()