
NUCLEAR SHELL MODEL
The shell model of nuclei says that, just like electrons around atoms, the nucleons within nuclei are in shells, and that nuclei with completed shells are more stable than those which have in-complete shells. This is only a model, and does not mirror exactly what happens within nuclei, but it is a good guide. Real nuclei are more complex than this simple model would suggest.

FIRST SHELL
The first complete nuclear shell happens when nuclei have four nucleons. However, it cannot be four protons and neither, it is thought, can it be four neutrons (but see 'Element Zero'). The four nucleons could be two neutrons and two protons (helium-4 nucleus - stable, aka alpha particle). Helium-4 is doubly magic. Or they could be three neutrons and one proton (hydrogen-4 - beta unstable) or its mirror analogue with 3 protons and one neutron (lithium-4 - inverse beta unstable). These last two are extremely unstable and decay within less than 10-23 seconds because they have either far too many protons for the number of neutrons, or vice versa. For stability, light isotopes require approximately a 1:1 neutron to proton ratio. The inner nuclear shell is complete when there are 4 nucleons within it, and these clump together at the four corners of a tetrahedron.
Shown is a 3D-stereo projection of the first shell, a tetrahedron. The placement of the nucleons is at the (four) apexes, numbered '1'.
(see 'Magic Numbers').
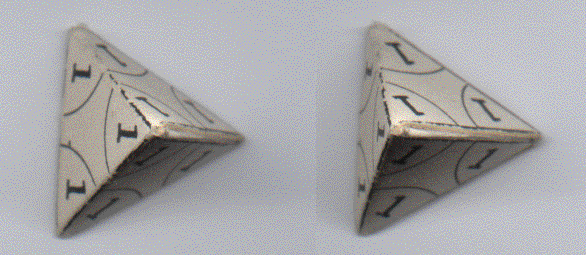

SECOND SHELL
The second nuclear shell has an extra 12 nucleons within it, and these surround the four nucleons of the first shell, making 16 nucleons in all. An example would be oxygen-16, which is doubly-magic.
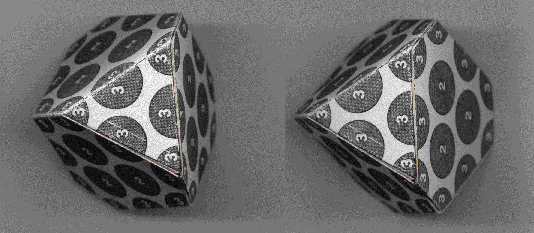
The 3D-stereo model of the second shell shows the position of the nucleons inner shell (numbered '1') and the nucleons of the second shell (numbered '2') which are situated at the 12 corners of a truncated tetrahedron. Note that the nucleons of the first shell occupy the centre of the faces of the senond shell.


THIRD SHELL
The third nuclear shell comprises an extra 24 nucleons. Again, 12 of these are situated at the 12 corners of an outer truncated tetrahedron, with the remaining 12 taking up positions in the middle of the edges of the truncated tetrahedron. This is best illustrated by the 3D-stereo scan below. Here the nucleons belonging to the third shell are numbered '3'. The 12 nucleons of the second shell occupy the 4 faces of the tetrahedron, three on each side arranged triangularly. The 4 nucleons of the first shell are hidden from view deep within. With the third shell full, the isotope would have 4 + 12 + 24 nucleons, totalling 40 nucleons in all.
An example would be calcium-40 with 20 neutrons and 20 protons. Calcium-40 is stable, and doubly-magic. Another example would be argon-40, with 18 protons and 22 neutrons, this too is stable (but not magic). Potassium-40 would be yet another example (this has 19 protons and 21 neutrons. Being an odd/odd nuclei this is not stable, it can decay by either beta decay or inverse beta decay)

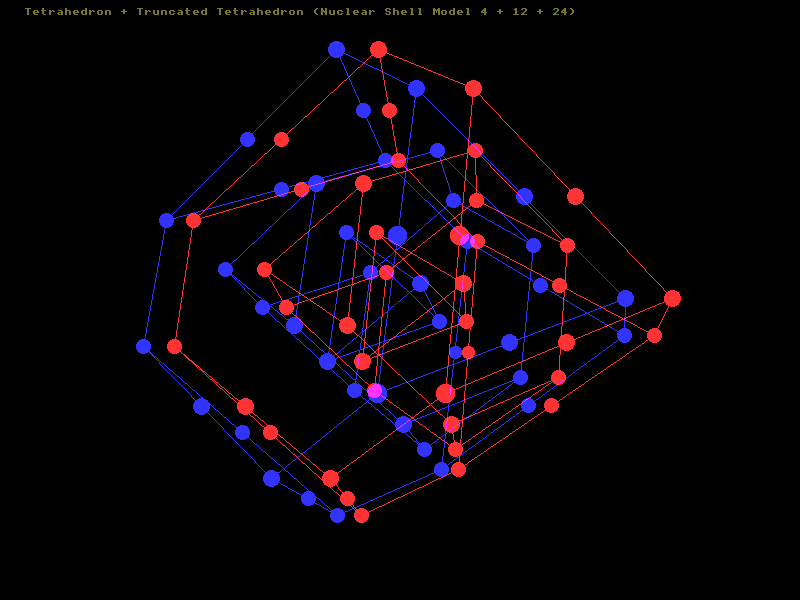
3D-Stereo Nuclear Shell Animation Showing 3 nested shells [Pop Out Page]

FOURTH SHELL (??)
Presumably the fourth shell would have an extra 36 nucleons, arranged in a similar truncated tetrahedron, making the total 4 + 12 + 24 + 36 = 76. This is where the model starts to break down. This does not correspond to any observed sum of magic numbers, the magic numbers being 2, 8, 20, 28, 50, 82 after which the magic number is different for protons and neutrons. For neutrons, the next magic numbers are 126 and 184; for protons it is 114. Large stable nucei often consist of smaller subgroups of nucleons, which are themselves stable.




![]()



![]()

![]()
![]()