

![]()
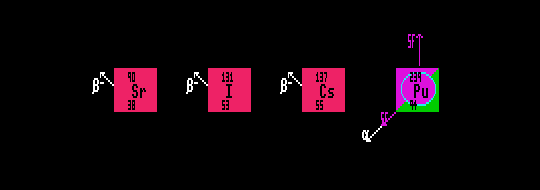
The volatile radionuclide, caesium-137, a beta emitter with halflife of 30 years, is a fallout product resulting from atmospheric nuclear tests. Upland areas of Britain are still badly contaminated with caesium-137 resulting from the fire at the Chernoble nuclear power station in 1986, preventing human consumption of contaminated sheep.
Plutonium-239 is created in fast breeder nuclear reactors by the ton and is a very serious hazard due to its high alpha activity and its' selective absorption by bone marrow. It now contaminates the whole Earth through atmospheric testing of nuclear weapons and accidental releases from nuclear power stations. Pu-239, halflife 24,100 years needs waste management for 250,000 years, but 500,000 years would be better as there is now so much of it around! After half a million years, there will be just one millionth of that isotope left.
Hundreds of millimetre sized particles of Pu-239, each one if ingested, could kill a person within a week, contaminate Dounreay beach through an accident at the Dounreay nuclear reactor in 1960: The operators of the nuclear plant were dumping radioactive waste plutonium containing other nuclides down an old dis-used mine-shaft. One day, they also dumped some sodium (used as coolant) down the shaft, which happened to be waterlogged. There was a large explosion as the sodium re-combined with water forming sodium hydroxide, pulverising and blasting the radioactive waste up the shaft and far asunder. These people are 'nuclear scientists'! The resultant contamination of the beach was kept quiet for years.
![]()
![]()