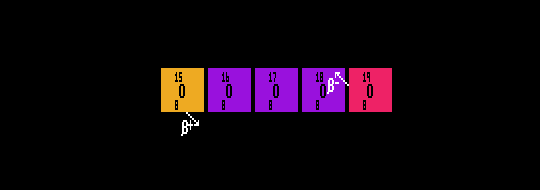
An element can exist as a variety of different isotopes, some stable against radioactive decay, others unstable and liable to emit various sub-atomic particles at any time. These particles could be protons, neutrons, electrons, positrons, neutrinos, gamma rays, or alpha particles. The element with the greatest number of known isotopes is Xenon with 35. For any given element, new isotopes are continually being found (produced in high energy particle accelerators), usually with shorter and shorter half-lives. Those in the Segre chart left blank are unknown, but if produced, may well have a significant half-life. On the Segre chart, the isotopes lie on a horizontal strip of the chart.
Shown are five isotopes of Oxygen, an inverse beta decayer (positron emitter), O-15; three stable isotopes, O-16, O-17 and O-18; and a beta decayer (electron emitter), O-19.
![]()