Shown is an animation of the radioactive decay of a red substance into a stable blue substance, along with a graph of the percentage abundance of the original substance plotted against time. Notice how the percentage abundance of the red substance changes with time, and in about 10 halflives virtually nothing is left of it. The growth in the blue substance is exponential and complimentary to the exponential decline in the red substance. The sound ticks are a representation of the detection of radiation from a decaying atom by a Geiger counter.
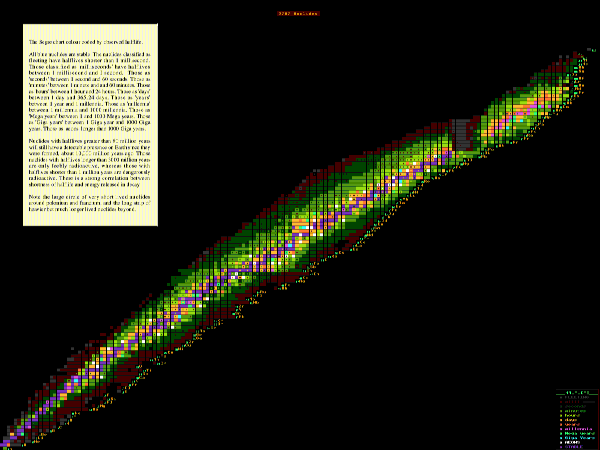
Nuclides with short halflives are highly radioactive and emit intense radiation but for only a short time, whereas nuclides with a long halflife are only weakly radioactive but will continue emitting it over long periods. Generally, short halflife nuclides are the more dangerous (if still present) whereas long halflife nuclides are only mildly dangerous but will be around for ages. This apparent inverse relationship between halflife and harmfulness is not that simple since it takes no account of the type of radiation emitted (gamma, beta, alpha etc) or of the energies of the emitted particles (the more energetic, the more damaging. Generally, charged radiation, like beta rays or positrons, is more harmful than neutral radiation, like neutrons or neutrinos, but again it dependes upon the energy of such radiation, and how strongly it interacts with matter. Neutrinos are safe because hardly any interact with matter).
![]()
![]()