
The nucleus consists of a number of protons (with positive charge) and (usually) an equal or greater number of neutrons (without charge) all held together against the electrostatic repulsion of the like-charged protons by the strong nuclear force. The neutrons serve to dilute the mutual coulombic repulsion between the protons.
The identity of the element is determined by the number of the protons within the nucleus (the Atomic Number, Z). For hydrogen, Z=1 whilst for lawrencium Z=103. The atomic number of an element is shown in the bottom left hand corner of the chemical element symbol. The atomic weight, being the sum of the number of protons and neutrons within the nucleus, is shown in the top right hand corner of the chemical symbol. There are over 100 different atoms, or elements, each with differing chemical and nuclear properties.
The diameter of an atom is typically 300 pico metres whereas that of the nucleus is very much smaller, about 3fm. Ordinarily, a single atom is so small that it is impossible to see, being much smaller than the wavelength of light, but under special circumstances, held stationary in the middle of a darkened evacuated chamber by two laser beams, a single atom is visible to the naked eye, though it appears much bigger than it actually is due to the finite resolution of the eye. Such an atom of barium, called 'Astrid', was studied recently.

If that same barium atom illuminated by a laser beam has a mirror positioned behind it, then the emitted light from the atom interferes with its' own reflected light such as to contrive to hold the atom stationary in a potential well: if the atom moves off-centre, then it scatters more light from the brighter region of the interference pattern, and less light from the darker region, creating a slight force that moves the atom back into the minimum. The atom thus becomes trapped by its' own reflection.
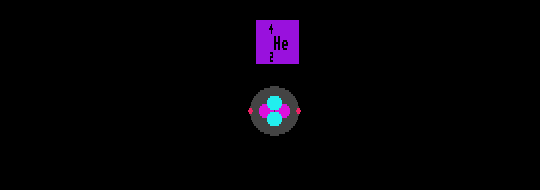
The stable Helium-4 nuclide is depicted with a representation of its structure, showing the alpha particle nucleus containing two neutrons and two protons and the two outer electrons. (Not to scale!)
![]()