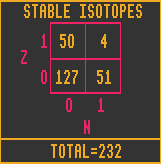
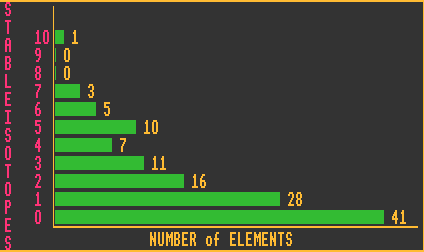


The bottom chart shows the number of elements that have a certain number of stable isotopes. Thus there are 3 elements with 7 stable isotopes and 41 elements with no stable isotopes (Although a lot depends upon your definition of stable. If an isotope has a halflife of 1010 years or more, do you class it as stable, or not? Here the unconditionally stable definition has been used). The only element with ten stable isotopes is tin, which is also the element with the greatest number of stable isotopes.
As of the year 2000 AD, of the known Elements, 75 have at least one stable isotope, and 41 (includes transuranic elements) have none.
![]()