
|
HELP on IONIZATION ENERGIES (in eV, electron Volts)
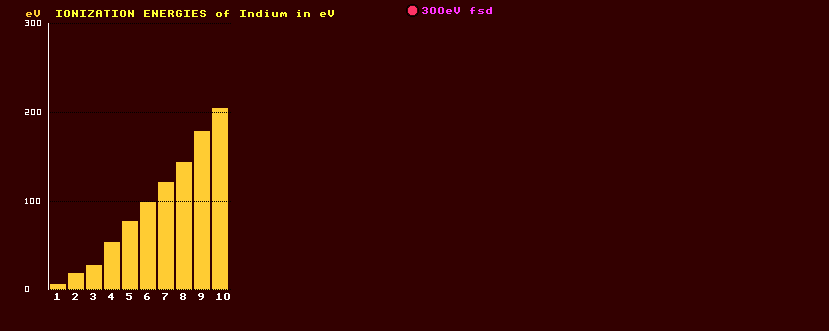
The Nth ionization energy is the energy required to remove one extra electron from an ion that has had (N-1) electrons removed already. It takes successively greater energies to strip more and more electrons from the atoms. This is because, as further negatively charged electrons are removed from the atom, it becomes progressively more positively charged and clings tenaciously onto its remaining electrons with greater and greater attractive force. Which group of elements has the highest Nth order ionization energies is determined by whether the (N-1)th ion has a closed shell. Hence the sudden step in ionization energy as N increases, which is particularly noticeable for those elements with atomic number below and including sodium. Please note the change in scale from 1500eV fsd to 300eV fsd between those elements below element number 20 (calcium) and those above.
|
![]()