
|
Radioactive substances decay when the atoms within it undergo disintegrations. The activity of a radioactive substance is defined as the number of disintegrations taking place per second in a sample of the radioactive substance. A Becquerel is a unit of activity, measured in disintegrations/second. Note that no mass is specified, it is the activity of a sample. Obviously, the bigger the sample, the larger the activity, so to normalise the data, I have quoted the activity in becquerels per kilogram.
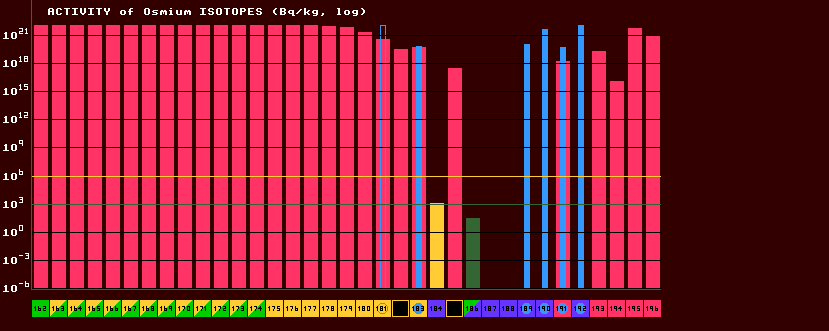
The activity per kilogram of a substance is inversely proportional both to the halflife and also to the isotopes atomic mass. Another unit of activity is the Curie, which is equal to 37×109 Becquerels. A Curie is a rather large unit as far as normal radiation laboratory work is concerned, where a milliCurie serves much better. A 1 gram mass of radium-226 gives off approximately 1 Curie of activity, or approx 37×1012 becquerels/kg. For those isotopes which have halflives much less than about 200 seconds, the activity per kilogram is absolutely enormous, greater than 10×1021Bq/kg. No man could survive exposure to even a gram of such a source. Legally, any radioactive substance with an activity of more than 400 Bq/kg is deemed radioactive. Any with an activity of more than about a million bequerels is likely to be dangerous. How dangerous, depends upon many other factors: the type of particle emitted; the energy of those particles; the mass of the source; the distance between you and the source; your exposure time to the source; your personal susceptiblity to that radiation (alcohol may offer some protection from radiation) and whether it is within your own body! For this reason, isotopes exceeding the orange line at 106 Bq/kg are shown in red, those below this but above the green line at 103 Bq/kg in orange, and all below this line in green. But remember, there is no safe 'dose' of radiation! The activity of metastable isotopes is shown in cyan; solid bars for internal transition isomers, and hollow bars for those that decay by other means, eg. beta or inverse beta decay, etc. Osmium is shown in the above example. Five isotopes are totally stable (blue) (Os-187 to Os-190 inclusive and Os-192). A sixth isotope, Os-186, is slightly alpha-radioactive (green/blue) but it's activity (dark green bar) is very low; there is a seventh isotope (Os-184) that is shown as stable (blue) but has a slightly higher activity just in the orange region. Osmium is perfectly safe to handle, it can be found in 'osmiridium' pen nibs. There are three stable isotopes of osmium that can be excited to metastable states (thin cyan bars), Os-189, Os-190 and Os-192, and these three have dangerously high activity. But these activity bars are for pure isotopes! Elemental osmium comprises seven stable plus semi-stable isotopes, and this has to be taken into account. When the isotopic composition of elemental osmium is studied, it is found that the (slightly) radioactive isotopes (Os-184 and Os-186) are present in negligible proportions. [For Isotopic composition of osmium see graph below]. Taking this into consideration, it can be seen that elemental osmium presents little or no radioactive risk at all.
To provide further evidence that elemental osmium is radiologically perfectly safe, the halflife chart for osmium is presented below. This shows that the two semi-stable isotopes have extremely long halflives, much greater than 1013 years! Long halflives mean low activity.
|
![]()