
|
96 CURIUM Cm (Madame Curie, physicist)
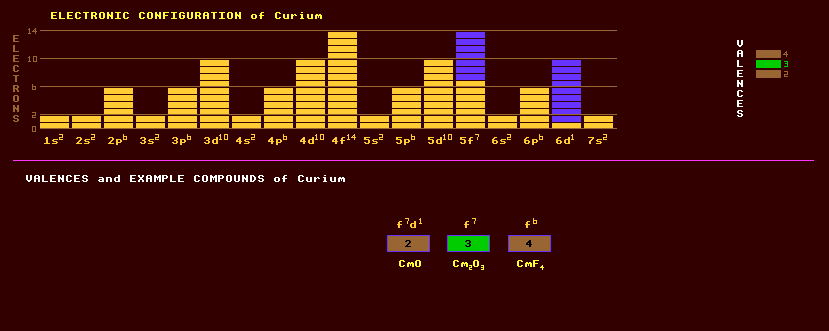
An artificially produced, highly unstable, transuranic rare earth element of the actinide series with no detectable natural occurrence on Earth. It is a radioactive silvery metal, attacked by oxygen, steam and acids but not by alkalis. Curium exhibits three valences, +2, a commonly expressed +3, and +4. Amongst the compounds that have been prepared are curium monoxide, CmO, the sesquioxide, Cm2O3, and the dioxide, CmO2. Curium hydroxide, Cm(OH)3, is known as are the following halides: curium trifluoride, CmF3, trichloride, CmCl3, and tetrafluoride, CmF4. Most trivalent compounds of curium are faintly yellow in colour. Curium is similar to gadolinium, its rare earth lanthanide analogue, but it has a more complex crystal structure. The magnetic susceptibilities of curium trifluoride and gadolinium trifluoride are of the same magnitude, providing direct evidence for assigning the 5f7 electronic configuration to curium. There are no natural sources of curium. Curium-242 and curium-244 are produced commercially by the bombardment of plutonium-239 with neutrons within nuclear reactors, and then extracted during nuclear reprocessing. Curium-242, generates about 3 watts of heat per gram, compare to just half a watt for plutonium-238, suggesting uses as an isotopic power source, but it has a short halflife of only 168 days. Small quantities of curium are also produced incidentally in nuclear explosions, and occur in nuclear fallout. The longest lived isotope of curium is curium-247 with a longish halflife of 15.6 Million years decaying by alpha decay into the beta decaying plutonium-243 which has a halflife of 5 hours. The halflife of curium-247, although longish, is short compared to the age of the Earth, and all the curium-247 that was present at the birth of the Earth has long since decayed. Traces of curium may exist within ores of Uranium in secular equilibrium as a result of neutron capture and beta decay processes, but has never been detected. Curium is absorbed into the body and accumulates in the bones destroying bone marrow and causing leukaemia, thus representing a severe radiological hazard. Altogether, 16 isotopes of curium are known, all radioactive, and ranging from the alpha/inverse beta decaying curium-236 to the beta decaying curium-251 which has a halflife of 16.8 minutes. Claim to fame:
|
![]()