
|
95 AMERICIUM Am (America)
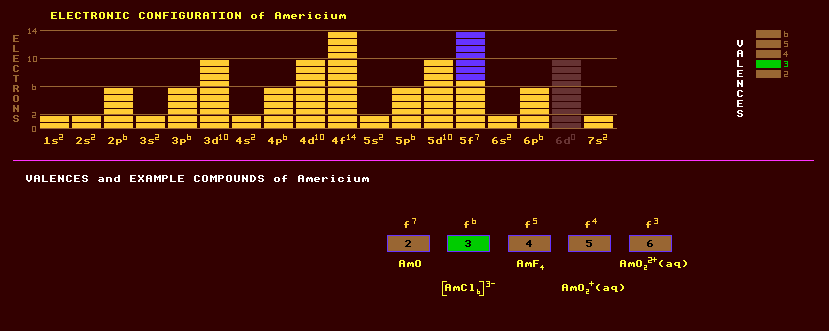
An artificially produced, highly unstable, transuranic rare earth metallic element of the actinide series with no detectable natural occurrence on Earth. Americium is formed by in a nuclear reactor as a by product of fission reactions, which release neutrons. The neutrons are captured by plutonium, one of the fuels (and itself a product of neutron capture by uranium), forming americium. Small quantities of americium are separated from nuclear waste during nuclear re-processing. Americium exhibits a range of valences from +3 to +6, with +3 being the most commonly exhibited. Among the many compounds prepared are the oxides americium monoxide, AmO; the sesquioxide, Am2O3; and the dioxide, AmO2. The halides include americium dichloride, AmCl2, the trifluoride, AmF3, and trichloride, AmCl3; the tetrafluoride, AmF4, and the complex ion [AmCl6]3-. Americium metal is prepared either by reducing americium trifluoride, AmF3, with barium vapour at between 1000 to 1200 Celsius, or by reducing the dioxide, AmO2, with lanthanum metal. Americium is a white metal and is more silvery than plutonium or neptunium prepared in the same way, but tarnishes slowly in dry air. Americium is more malleable than uranium or neptunium. Two allotropic forms of americium are known: the stable alpha form is double hexagonal close packed; the beta form is loosely packed and cubic. Americium-241 and americium-243 are the two most important isomers of americium, having the longest halflives. Americium-241, an alpha particle emitter with a half-life of 432 years is used as an ionization source in smoke detecting alarms, and is poisonous on account of its radioactivity, the maximum permissible burden in humans is 30 nano Curies. The alpha activity from americium-241 is three times greater than that from radium. The longest lived isotope of americium is americium-243 with a halflife of 7370 years decaying by either spontaneous fission or by alpha decay into the beta decaying neptunium-239, which has a halflife of 2.4 days. Altogether, 13 isotopes of americium are known, all radioactive, and ranging from the alpha/inverse beta decaying americium-232 which has a halflife of 54 seconds to the beta decaying americium-247 with a halflife of 22 minutes. Claim to fame:
|
![]()