
|
94 PLUTONIUM Pu (Planet Pluto)
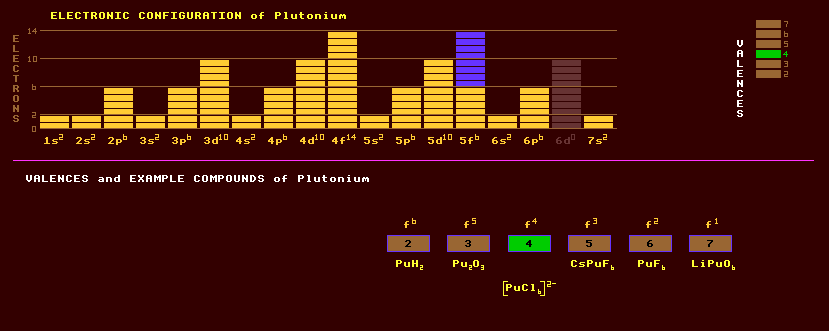
Plutonium is a metallic element belonging to the actinide series and does not occur naturally on Earth except in trace amounts in uranium ores because it is highly radioactive with no stable isotopes. Plutonium is warm because of its radioactivity, is silvery, tarnishes in air to a yellow colour with formation of an oxide film, and dissolves in concentrated HI, HCl, and HClO4 acids. On heating from 395 Kelvin to 753 Kelvin plutonium goes through six metallic phases: two monoclinic, an orthorhombic, a face centred cubic, a tetragonal, and a body centred cubic phase, each with abrupt transition temperatures accompanied by sharp changes in density ranging from 16.0 to 19.86. The face centred cubic form has the least density. That and the tetragonal phase above it have negative temperature coefficients of thermal expansion, they contract on heating. In order to eliminate some of these phase changes for bomb-grade plutonium, it is alloyed with 1% gallium to stabilise it over a wide range of temperatures. Unfortunately this makes it's peaceful destruction in nuclear reactors problematic as the gallium will react with the zirconium cladding of fuel rods, and must be removed. Plutonium exhibits a range of valences from +2 to +7 with +4 being the most commonly expressed. Despite belonging to the actinides, plutonium shares a few chemical similarities to the group 8 elements iron, ruthenium and osmium. In aqueous solutions, the +3 valency is lavender in colour, the +4 yellow brown, the PuO+ ion pink and the PuO2+ orange. Among the known oxides are plutonium monoxide, PuO, the sesquioxide, Pu2O3, and the dioxide, PuO2. The halides prepared include plutonium triiodide, PuI3, the trichloride, PuCl3, the trifluoride, PuF3, the tetrafluoride, PuF4, and the hexafluoride, PuF6. Oxyhalides include PuOCl, PuOBr, PuOI. Other compounds are plutonium carbide, PuC, the nitride, PuN, the disilicide, PuSi2, the hydride, PuH2, CsPuF6 and Li5PuO6. Plutonium-239 is created by man in a fast breeder nuclear reactor: The power generating core of a nuclear reactor comprising uranium-235 enriched with plutonium-239 decays by neutron induced fission producing a self sustaining chain reaction of neutrons. A blanket of relatively non-fissile uranium-238, which is in plentiful supply, surrounds the nuclear core absorbing any escaping neutrons and producing uranium-239, which eventually produces plutonium-239 by the chain of neutron capture and beta decay events as described above. The reactor miraculously makes its own fuel! By 1982, 300,000 kilograms of plutonium-239 had been made. Plutonium-239 is highly fissile (easily undergoing neutron-induced fission) and great precautions must be taken to prevent the accidental formation of the critical mass, which also depends on shape, but is only 5.6kg for a sphere. But as little as half a kilogram is sufficient for criticality in aqueous solutions of plutonium-239 compounds, because the water moderates (slows down) the released neutrons making them more likely to induce further fission. Plutonium-239 is used in nuclear bombs, with one kilogram releasing as much nuclear energy as 20,000 tons of TNT, or 22 Gigawatt hours of energy. The greater fissionability of plutonium-239 makes the design of efficient nuclear weapons more difficult, because some can undergo neutron induced fission prematurely before the critical mass has fully assembled vaporising the rest before the chain reaction has finished. The incidental inclusion of any plutonium-240 is particularly troublesome in this way, and must be avoided. Because the plutonium-240 is made by the neutron bombardment of plutonium-239 within the fast breeder reactor itself, production of plutonium-240 cam be minimised by withdrawing the fuel elements earlier than necessary for their use in power production. Plutonium-239 is a very dangerous radiological hazard due to its high alpha radioactivity and its selective absorption by bone marrow. It now contaminates every square inch of the Earths' surface, caused by man-made nuclear explosions and accidental releases from nuclear power stations. Plutonium-239 has a longish halflife of 24,100 years, and as nuclear waste, must be managed for 250,000 years. If inhaled, just one gram of plutonium-239 dust possesses enough radioactivity to kill thousands of people. But chemically, it should be as harmless as other, non-radioactive, rare earth elements like cerium. If ingested by the body, plutonium concentrates in the bones where it is likely to cause leukaemia by irradiating the stem cells in bone marrow. The dangers posed by possible terrorist acquisition of some of the vast stores of plutonium-239 have prompted investigation into the possibility of using mixed oxide, MOX, fuels in nuclear power stations. It is proposed that by using a mixture of uranium and plutonium oxides, UO2 and PuO2, the risk is minimised since the terrorists would need to chemically process the fuels before use in weapons. Disposal of spent MOX fuel by glassification and subsequent entombment is now thrown into doubt by the recent, 1999, discovery that the yellow water-insoluble plutonium oxide very slowly reacts with oxygen in the presence of water forming a green higher oxide with an as yet unknown composition, but which is highly soluble in water. The subsequent dissemination of plutonium through groundwater would be far faster than was at first envisaged. All plutonium that was formed from the supernova explosion which occurred 150 Million years before the solar system coalesced (and was the cause of its coalescence) has long since decayed, because the longest lived isotope of plutonium (plutonium-244) has a halflife of just 82 million years. The traces of plutonium found in uranium ores on Earth today are being continually created by neutron capture events within the ore itself: the spontaneous fission of uranium-235 releases a few neutrons which atoms of uranium-238 may capture, producing uranium-239, (halflife 23 minutes) then decays by beta decay to neptunium-239 (halflife 2.35 days) which decays again by beta decay to plutonium-239. For any one atom the probability of this chain occurring is incredibly tiny, so the amounts produced are minute. The dismantling of nuclear weapons containing metallic plutonium merely involves re-shaping the 'pit' from its highly classified shape into any other shape. This process previously generated huge amounts of radioactive waste as the plutonium metal was dissolved in acid and then processed back into metallic plutonium. A new faster process which generates no radioactive waste is to react the plutonium metal with hydrogen at reduced pressure, forming plutonium hydride, which is a powder and falls off the pit. Metallic plutonium is re-generated by heating the hydride, and the evolved hydrogen gas is recovered for re-cycling more plutonium pits. Plutonium-238 is obtained by the neutron bombardment of neptunium-237 within a nuclear reactor. It is an alpha emitter with no accompanying gamma radiation, and is thus relatively safe to use as a heat source in thermoelectric power supplies, as the alpha rays are easily shielded by an enclosure. The halflife of plutonium-238 is 87.7 years, giving the device a long life, and produces about half a watt of heat per gram. It was used to power the seismic monitors left on the Moon by the Apollo missions, and has been used, well shielded, in cardiac pacemakers and as a direct heat source in deep-sea divers suits to keep them warm. Altogether, 16 isotopes of plutonium are known, all radioactive, and ranging from the alpha decaying plutonium-230 to the beta decaying plutonium-246 which has a halflife of 10.8 days. Plutonium-244 has the longest halflife (of 82 Million years), has now been found in nature, and decays by alpha decay into the beta decaying uranium-240 which has a halflife of 14 hours. The plutonium isotope with the second longest halflife is plutonium-242, at 380,000 years. Claim to fame: The most poisonous substance. Plutonium also has a greater number of metallic phases than any other element, six in all, and is also the only element to have a negative temperature coefficient of resistance (of -870ppm/ºC).
|
![]()