
|
93 NEPTUNIUM Np (planet Neptune)
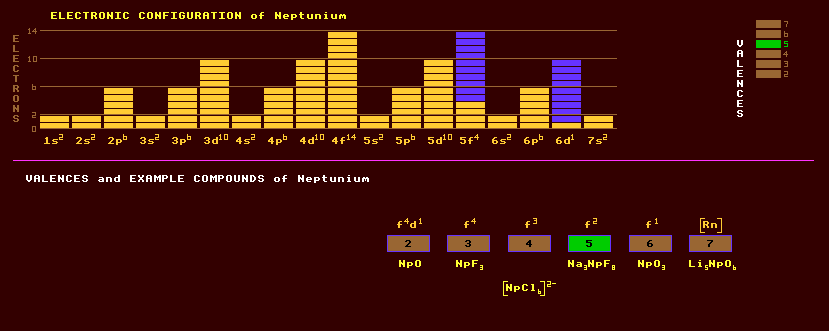
Neptunium is an element belonging to the actinide series and does not occur naturally on Earth except in trace amounts because it is highly radioactive with no stable isotopes. Neptunium is a metal with a silvery appearance, and is chemically reactive. Neptunium occurs in trace amounts in uranium ores due to the transmutation of uranium and thorium by the neutrons given off by the spontaneous fission of uranium. Neptunium is attacked by acids, steam and oxygen, but not alkalis. It is known to exist in three allotropic forms: the room temperature stable alpha-neptunium which is orthorhombic and has a specific density of 20.25; above a temperature of 551 Kelvin beta-neptunium is stable, tetragonal, and has a density of 19.36; and above 850 Kelvin, gamma-neptunium is stable, cubic , and has a density of 18.0. Neptunium has seven oxidation states from +2 to +7, the most common of which is +5. Despite belonging to the actinides, neptunium shares a few chemical similarities to the group 7 elements manganese, technetium and rhenium. Compounds in oxidation state Np+3 are pale purple, those in oxidation state Np+4 are yellow green and is analogous to those of the rare earth Pm+4; those of NpO2+ are greeny blue whilst those of NpO2++ are pale pink. These latter oxygenated species have no analogues to the rare earths which exhibit only simple ions of the (+2), (+3) and (+4) species. Known compounds include the halides NpF3, NpF4, NpF5, NpF6, NpCl4, NpBr3, NpI3 etc, various oxides such as Np3O8, NpO2, NpO; and other compounds such as CsNpF6, Na3NpF8 and Li5NpO6. Altogether, 17 isotopes of neptunium are known, ranging from the alpha decaying neptunium-226 which has a halflife of just 30 milliseconds to the beta decaying neptunium-242 with a halflife of 5.5 minutes. The longest lived isotope of neptunium, present in only trace amounts on Earth, is neptunium-237 with a longish halflife of 2.14 Million years decaying by alpha decay into the beta decaying protactinium-233 which has a halflife of only 27 days. It is, however, found naturally on the moon, where it is produced by cosmic ray bombardment of moon rocks. Neptunium-237 can be used in neutron detection instruments. Neptunium-237 and neptunium-239 are made in gram quantities in nuclear reactors as a by product of the production of plutonium. Neptunium-239 has a much shorter halflife of only 2.35 days. The relatively small amounts of neptunium produced in nuclear reactors and in the fallout from nuclear bombs means that it is an un-important radiological hazzard in comparison to other radionuclides. Claim to fame: Neptunium has the highest liquid range of 3262ºC of any element. Of the actinides, neptunium is the only one not to be poisonous to humans.
|
![]()