
|
92 URANIUM U (Uranus, planet)
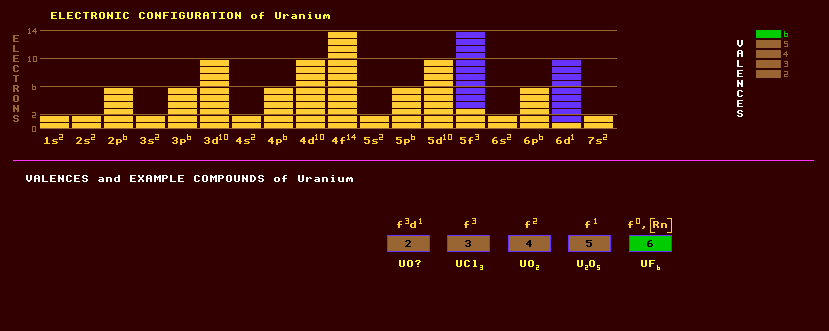
A radioactive, silvery, malleable and ductile rare earth element of the actinide series that exists in three allotropic forms: stable alpha uranium which is orthorhombic, which transforms into the tetragonal beta uranium above 668ºC, and into the body centred cubic gamma uranium above 774ºC. Naturally occurring uranium consists of three isotopes: uranium-238 which comprising 99.3% of naturally occurring uranium, is non-fissile, has the longest halflife (4,460 Million years) and decays by alpha decay into the beta decaying thorium-234 which has a halflife of 24 days. The halflife of U-238 is of a geological time scale and has been used to measure the age of the Earth and rocks. With such a low activity (long halflife) U-238 is relatively radiologically harmless. Uranium-235, which is fissile and comprises just 0.7% of uranium, has a halflife of only 704 million years and can decay by either spontaneous fission into a variety of daughter products emitting several neutrons or by alpha decay into thorium-231, a beta emitter with a halflife of 1 day. The only other isotope occurring naturally is uranium-234 at 0.005%, an alpha emitter with a halflife of 250,000 years. The U-235 can be separated from the U-238 by passing uranium hexafluoride gas (which is gaseous above 65 Celsius) through many hundreds of centrifuges, taking advantage of the slight mass excess of U-238 over U-235. The remaining U-238 is said to be depleted uranium, and can be used as armour plate on tanks, as ballistic tips on armour piercing shells, or to generate plutonium-239 in fast breeder reactors (see plutonium). The U-235 is fissile and used in nuclear bombs and power generating reactors, but care must be taken not to exceed its critical mass, which also depends on shape, otherwise a runaway nuclear chain reaction results as the neutrons released in spontaneous fission induce the fission of more U-235. For use as a nuclear fuel, uranium-235 is concentrated from its normal isotopic abundance of 0.72% to 3%, when it is known as enriched uranium. Left at a concentration of 0.72%, the critical mass is enormous and would require a good moderator to initiate a nuclear runaway reaction. Water makes a good moderator, and the critical mass of a solution of 100% abundance uranium-235 is just 0.8kg. A uranium ore deposit in Oklo, Russia, where the U-235 isotopic abundance is now only 0.4%, must at one time in the geological past when the abundance exceeded 3%, have gone critical consuming some of the U-235. This reaction was also moderated by water. (See Oklo allobar - !Nuclides). Uranium-235 decays by a number of steps, called the U-235 series, to eventually reach the stable isotope lead-207.Uranium-236 decays by the different U-236 decay series to become stable lead-208. Uranium-238 (and U-234) decays by the different U-238 decay series to become stable lead-206. But uranium-237 decays into stable bismuth-209. See !Nuclides and plot the decay series for yourself. Uranium exhibits five valences ranging from +2 to +6, the most common of which is +6, and, despite belonging to the actinides, shares certain chemical similarities to group 6 elements, chromium, molybdenum and tungsten. Amongst the prepared compounds are the following: uranium dioxide or yellow cake, UO2; a pentoxide, U2O5; a trioxide, UO3; and U3O8. The halides include uranium trifluoride, UF3 and trichloride, UCl3; uranium tetrafluoride, UF4 and tetrachloride, UCl4; uranium pentafluoride, UF5; pentachloride, UCl5, and pentabromide, UBr5; uranium hexafluoride, UF6, and hexachloride, UCl6. Uranium carbide, UC2, yields a mixture of hydrocarbons on hydrolysis with water, namely acetylene, C2H2; ethylene, C2H4; and hydrogen, H2. The main ores are highly radioactive and very heavy: Uraninite, UO2 and pitchblende, U3O8, are hard and black; carnotite, K2(UO2)(VO4)2·2H2O, soft, yellow and powdery; thorianite, (Th,U)O2, black and cubic; and samarskite, (Y,Ce,U,Ca,Pb)(Nb,Ta,Ti,Sn)2O6 which contains many valuable elements. Phosphates contain great reserves of uranium: the lime yellow autunite, Ca(UO2)2(PO4)2·10-12H2O fluoresces yellow green in ultraviolet light and has a mica-like cleavage, as does the related grass green torbernite, copper uranite, Cu[UO2PO4]2·8-12H2O and yellow uranophane, CaH2[UO2SiO4]·5H2O which are rarer. Zippeite, U2SO10·3-6H2O, a complex hydrated uranium sulphate which results from the decomposition of pitchblende and occurs as a powdery bright yellow coating was once used as a yellow pigment in paints until it's radioactivity was discovered. Previously ores containing less than 0.5% of uranium were un-economic to mine, but now uranium can be recovered from low grade ores containing less than 0.08% uranium with the help of microbes. The slurry is mixed with ferrous iron sulphide which the microbe T.ferrooxidans converts to ferric sulphate. The acidic sulphate then dissolves the uranium compounds which can then be recovered by concentration and precipitation. Altogether, 18 isotopes of uranium are known, all radioactive, ranging from the alpha decaying uranium-222 to the beta decaying uranium-242. Just a trace of the isotope uranium-236, halflife 23.9 million years, exists on Earth, and is produced by neutron capture and s-processes within uranium ores having sufficient slow neutrons available. Claim to fame:
|
![]()