
|
91 PROTACTINIUM Pa (Greek: protos = first, + Actinium)
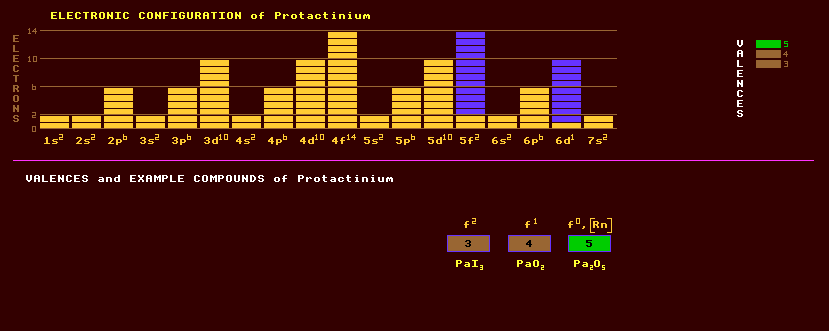
Protactinium is a silvery metal belonging to the actinide series and does not occur naturally on Earth except in trace amounts because it is highly radioactive with no stable isotopes. It is found in minute traces associated with uranium ores. It becomes superconducting below 1.4 Kelvin. It is attacked by acids, steam and oxygen, but not by alkalis. Protactinium exhibits three valences, +3, +4, and the commonly expressed +5. Despite belonging to the actinides, protactinium shares some chemical similarities to the group 5 elements vanadium, niobium, and tantalum. Amongst the compounds that have been prepared are protactinium triiodide, PaI3, the tetrafluoride, PaF4, and tetrachloride, PaCl4, protactinium pentafluoride, PaF5 and pentachloride, PaCl5, pentaiodide, PaI5, protactinium dioxide, PaO2, the pentoxide, Pa2O5, and the following complexes: [PaF6]-, [PaF7]2-, and [PaF8]3-. Some compounds are coloured. It can be purified by converting the oxide Pa2O5 to the pentaiodide PaI5, and decomposing the iodide on a hot filament heated electrically in a vacuum. In 1961 the UKAEA extracted 125 grams of protactinium by a 12-stage process from 60 tons of waste at phenomenal cost, constituting the Worlds biggest supply. It is thus one of the most expensive and rarest naturally occurring element. Protactinium-234 was the first isotope of protactinium to be discovered and is derived from the decay series of uranium-238. The longest lived isotope of protactinium is protactinium-231 with a relatively long halflife of 32,500 years decaying by alpha decay into the alpha/beta decaying actinium-227 which has a halflife of 22 years. Traces of protactinium-231 occur in pitchblende ores from Zaire, a uranium ore, at 1 part in 107. Since is is a product of the decay series of the relatively rare uranium-235, its concentration is very low. Protactinium-231 presents a severe radiological hazard similar to polonium. Protactinium has also been found in insignificant traces in sea water, a few parts in 1017 Altogether, 22 isotopes of protactinium are known, all radioactive, and ranging from the alpha decaying protactinium-215 which has a halflife of just 14 milliseconds to the beta decaying protactinium-238 which has a halflife of 2.3 minutes. Protactinium-231 is the longest lived isotope of protactinium, and has a halflife of 32,500 years. Claim to fame: The rarest and most expensive naturally occurring element. |
![]()