
|
90 THORIUM Th (Thor, Scandinavian God)
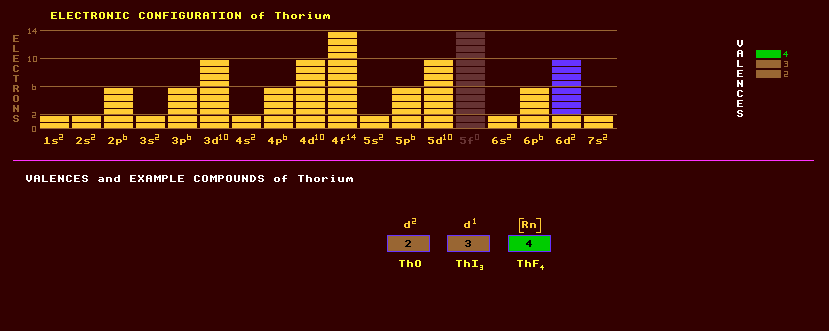
Thorium is an element belonging to the actinide series but although it is highly radioactive with no stable isotopes, some of its isotopes have very long halflives and do occur naturally. When pure, thorium is silvery white metal unaffected by air, but when contaminated by the oxide, it slowly tarnishes in air gradually turning black. Thorium is slowly attacked by water, and dissolves in hydrochloric acid. Its physical properties are greatly affected by contamination with the oxide, pure thorium is soft malleable and ductile. Thorium is dimorphic, changing from cubic to body centred cubic at 1400 Celsius. Thorium powder is pyrophoric, and turnings ignite if heated burning with a brilliant white light. Thorium, alloyed to magnesium, imparts strength and creep resistance at elevated temperatures. Thorium has a low work function, and about 1% of thorium alloyed into the tungsten filament of a thermionic valve lowers the work function of tungsten from 4.55eV to only 2.6eV, thereby allowing a reduction in working temperature from 2700C to only 1900C, with subsequent four-fold reduction in heater current required to heat the filament (it was later found that coating the filament in a metal oxide such as a mixture of strontium and barium oxides, the work function could be reduced to just 1.1eV, allowing filament temperatures to be reduced to just 700C). Thorium is used as a nuclear fuel in experimental reactors, as it gives more energy than does uranium, but there are difficult problems still to be overcome. The internally generated heat of the Earth is mostly from uranium and thorium decay. Thorium exhibits three valences, +2, +3, and the commonly expressed +4. Despite belonging to the actinides, protactinium shares many chemical similarities to the group 4 elements titanium, zirconium and hafnium. Compounds include thorium monoxide, ThO, the dioxide, ThO2, thorium hydride, ThH2, thorium triiodide, ThI3, and tetrafluoride, ThF4. Thorium nitrate mixed with 1% cerium nitrate is dried onto gas mantles: the thorium oxide, thoria, and cerium oxide, ceria, deposited in the mantle when first lit, emit a dazzling white light. Thoria melts at 3300 Celsius, the highest of all oxides, and is used to make high temperature laboratory crucibles; in high-dispersion glass for camera lenses and microscopes; and as a catalyst in petroleum cracking, and production of sulphuric and nitric acids. Thorium is about three times more abundant than uranium and occurs in the minerals thorite, ThSiO4, uranothorite (a mixed silicate of thorium and uranium) thorianite, ThO2.UO2, and at up to 9% in monazite sands with mixtures of other rare earths. Thorium-232, representing nearly all of naturally occurring thorium, is the longest lived isotope of thorium with a halflife of 14,100 Million years and decays by alpha decay into the beta decaying radium-228 which has a halflife of 5.76 years. It's long halflife has been used to date the Earth and rocks; it eventually ends up as lead-208 after six alpha and four beta decay steps, via the intermediate radon-220, or thoron, a gas presenting a dangerous radiological hazard. Thus thorium should be stored in well ventilated areas.
It has been suggested that thorium-232 could be used as a nuclear fuel and has the advantage over the presently used but relatively rare isotope of uranium-235 in that it is more abundant. Under neutron bombardment in a nuclear reactor, thorium-232 absorbs the neutron to become thorium-233, which in turn rapidly decays via the intermediary protactinium-233 into the fissile isotope uranium-233. It is the thus produced uranium-233 that would perhaps comprise the nuclear fuel of future nuclear power stations, which could be simultaneously used to breed more uranium-233 by surrounding the fuel with a blanket of thorium-232 to absorb stray neutrons. 232Th + n Altogether, 25 isotopes of thorium are known, all radioactive, and ranging from the alpha decaying thorium-212 which has a halflife of just 30 milliseconds to the beta decaying thorium-236 which has a halflife of 37.5 minutes. Claim to fame:
|
![]()