
|
9 FLUORINE F (Latin: fluere = to flow)
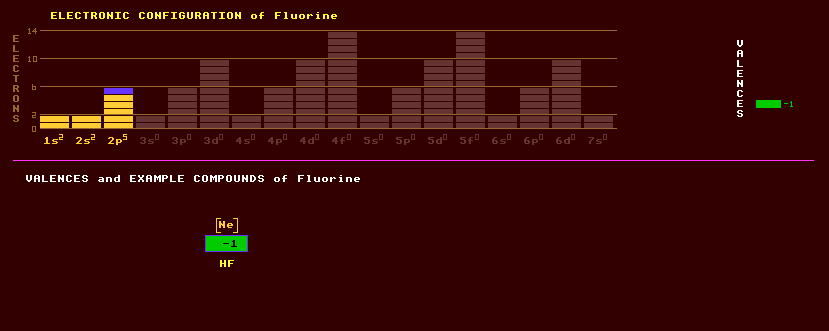
Fluorine is a highly reactive green monatomic gas with an irritating smell. It is the lightest member of the halogen group, group 17, to which chlorine, bromine, iodine and astatine belong. It has a valency of -1 and is the most highly electronegative of all the elements, which makes it highly reactive chemically, and is never found in its elemental state on Earth, but always combined with other elements. It has a small atomic size, but is larger than hydrogen. Fluorine is unaffected only by oxygen, chlorine and nitrogen but reacts explosively with hydrogen forming hydrofluoric acid, HF, used for etching glass. The principal ore of fluorine is fluorite or fluorspar, calcium fluoride, CaF2, found in Derbyshire in the Blue John mines, where the crystals are tinged purple by other minerals. Green, yellow and pink varieties of fluorite are also known. Blue John is also used decoratively as ornaments. The coloured varieties fluoresce strongly in ultraviolet light, hence the name. Fluorine is also present in cryolite, Na3AlF6, an ore of aluminium otherwise known as 'ice stone', a clear mineral almost invisible in water because of its similar refractive index. Fluorine is also present in the phosphate mineral fluoroapatite, Ca5(PO4)3F. Elemental fluorine is extremely poisonous, but combined with calcium in calcium fluoride, CaF2, or with tin as stannous fluoride, SnF2, it is added to toothpaste and to municipal water supplies to prevent tooth decay. The fluorine replaces the hydroxyl radical in hydroxyapatite, Ca5(PO4)3(OH), the main constituent of teeth and bones, forming fluoroapatite, which is more resistant to chemical attack or tooth decay. Fluoride levels in water up to 1ppm are thought beneficial by some, but in much higher concentrations fluoride is toxic, weakening the bones. Exposure to hydrogen fluoride, HF, produces painful slow healing skin burns, which should be treated with copious amounts of water followed by the application of calcium gluconate and an essential visit to the doctors. Combined with hydrocarbon in plastics, fluorine replaces the hydrogen, making the plastic more inert and raising the melting point. Thus PTFE (polytetrafluoroethylene, teflon) is so inert that nothing sticks to it and is used to coat non-stick frying pans, where its high melting point of 400 Celsius is usefully applied. Unlike the other halogens, fluorine has no analogue to the chloric, bromic or iodic acids and so does not form fluorates. Combined with sulphur it can form the poisonous and highly reactive gas sulphur tetrafluoride, SF4, and the inert gas sulphur hexafluoride, SF6, which is used to electrically insulate high-voltage circuit breakers in electrical transmission lines. Because the velocity of sound propagation is much lower in SF6, it is used in some double glazed windows to further increase acoustic attenuation. The gaseous uranium hexafluoride, UF6, is used to concentrate the uranium-235 isotope from the much more abundant uranium-238 for use in nuclear bombs, er, sorry, nuclear power stations. Magnesium fluoride, MgF2, was once used to optically coat glass to reduce reflection in camera lenses. Fluorine consists of just one stable isotope, F-19. In addition, 10 radioactive isotopes of fluorine are known, ranging from F-15 to F-25. Claim to fame: The most electronegative element, 4.1 units (Pauling), and the element with the shortest nearest neighbour at 144 picometres.
|
![]()