
|
89 ACTINIUM Ac (Greek: aktinos = beam or ray)
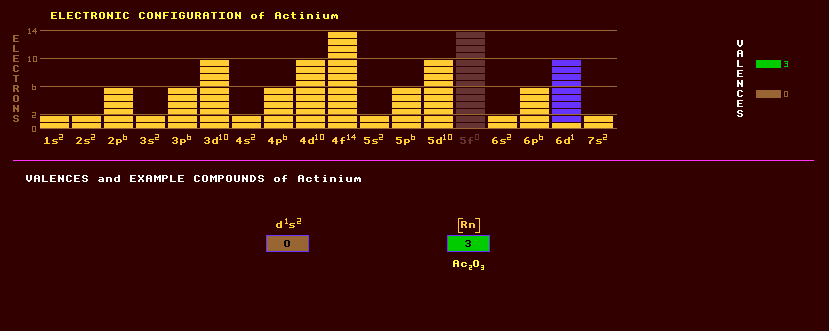
An artificially produced, highly unstable, transuranic rare earth element the first member of the actinide series. Traces of the longest lived radioactive isotope, actinium-227, are produced from the natural radioactive decay of uranium-235 via thorium-231 and protactinium-231. It is also produced by the neutron bombardment (and subsequent neutron capture) of radium-226. Actinium is a soft, silvery white metal which glows in the dark due to radioactivity. Actinium exhibits valency of +3, and the chemistry is similar to that of other rare earths, particularly lanthanum. The following compounds of actinium have been prepared: actinium oxide, Ac2O3, an insoluble in water actinium hydroxide, Ac(OH)3, and actinium hydride, AcH3. Actinium reacts with water evolving hydrogen gas and producing actinium hydroxide, Ac(OH)3. Actinium is chemically similar to the rare earths, particularly lanthanum, its lanthanide series homologue. The longest lived isotope of actinium, present on Earth in only trace amounts (0.2ppm in Uranium ores), is actinium-227 which has a half life of 21.8 years, and is a product of the radioactive decay of uranium-235. Actinium-227 can be obtained in milligram quantities by the neutron bombardment of radium-226, and decays by either alpha decay into the alpha/beta decaying francium-223 which has a halflife of 22 minutes or by beta decay into the alpha decaying thorium-227, which has a halflife of 19 days. Radium-223, halflife 11 days, is also one of the products of actinium-227 decay. All these decay products and more are in secular equilibrium with actinium-227. Purified actinium-227 comes into equilibrium with its decay products after 185 days, thereafter decaying according to its 21.8 year halflife. It is a powerful source of alpha particles, 150 times more active than radium and of value as a producer of neutrons. The alpha emitter actinium-228 is also present in trace amounts and has a halflife of just over 6 hours and comes from the radioactive decay of thorium-232, which has a halflife of 14,000 million years. Altogether, 26 isotopes of actinium are known, all radioactive, and ranging from the alpha decaying actinium-209 which has a halflife of just 100 milliseconds to the beta decaying actinium-234 which has a halflife of just 40 seconds. Claim to fame:
|
![]()