
|
88 RADIUM Ra (Latin: radius = ray)
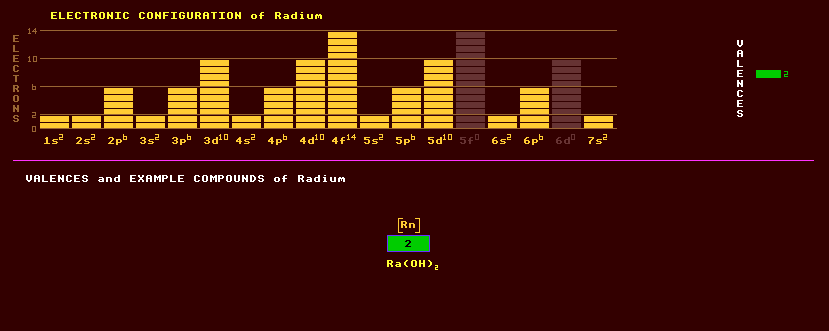
Radium belongs to the alkali earth metals along with beryllium, magnesium, calcium, strontium and barium. Radium does not occur naturally on Earth except in trace amounts from the radioactive decay of uranium, because it is highly radioactive with no stable isotopes. It is a pure brilliant white when freshly prepared, but blackens on exposure to the air due to formation of the nitride, and decomposes in water. Radium was discovered in 1911 by Madame Curie. Radium is used as a neutron source, and in the past together with a phosphor, usually zinc sulphide, in luminescent paint on watch dials. Radium colours a flame red. Radium and its salts are luminescent due to radiation. Radium emits alpha, beta and gamma radiation, and produces neutrons when mixed with beryllium. Radium exhibits only one valency, that of +2. The following compounds have been prepared: radium oxide, RaO, radium hydroxide, Ra(OH)2, and a complex [Ra(H2O}x]2+ which exists only in aqueous solution. Ores include uraninite, UO2; pitchblende (UO2,U3O8) at a concentration of 1 part in 7 million, and some carnotite sands, K2(UO2)2(VO4)2·H2O, and is also present in all uranium minerals from the radioactive decay chain of uranium-238 which produces radium-226, amongst other elements. It also occurs in broggerite, and cleveite. It is present in sea water at the insignificant dilution of 1 part in 1017, and concentrates in the bones of the human body where it is found at a level of a few parts in 1015. The longest lived isotope of radium is radium-226, a product of the decay series of uranium-238 (via an intermediary and much shorter lived uranium-234). It is present on Earth in only trace amounts at a concentration of 0.3mg per kilogram of uranium in uranium ores. It has a halflife of 1600 years and decays by alpha decay into the alpha decaying radon-226 (radium emanation), a gas with a halflife of 3.8 days which represents a dangerous radiological hazard. Therefore it should be stored in a well ventilated area. It was once used as the energy source (with zinc sulphide as the phosphor) in luminous dials on watches and aircraft instruments. One gram of radium releases 0.1 microlitres of radon gas per day, collected and sealed into files, is used to treat cancer. The Curie (Ci) is defined as the amount of radioactivity produced by one gram of radium-226, which produces 37 Giga becquerals (disintegrations per second). This level of activity is highly dangerous. The maximum permissible body burden of radium-226 is 7400 becquerals. Radium-226 in sufficient concentration to alert the World Health Organisation, has been found (AD 2004) in some brands of bottled mineral waters from France, Germany, Austria, Portugal and Hungary. People drinking a litre of the water from Hungary a day could breach the limit of 100 microsievetts a year recommended for drinking water. Altogether, 25 isotopes of radium are known, all radioactive, and ranging from the alpha decaying radium-206 with a halflife of just 400 milliseconds to the beta decaying radium-230 with a halflife of 1.5 hours. Claim to fame:
|
![]()