
|
87 FRANCIUM Fr (France)
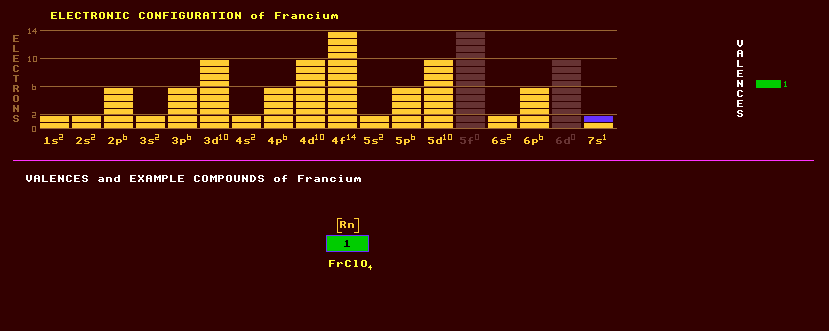
Francium is the last member of the alkali metals, which are: lithium, sodium, potassium, rubidium and caesium. Francium does not occur naturally on Earth except in trace amounts because it is highly radioactive with no stable isotopes. Francium is found in trace amounts in uranium minerals, but less than 30 grams are in the whole of the Earths crust at any one time. Francium exhibits only one valency, that of +1. An insoluble francium perchlorate, FrClO4 has been prepared. Chemically, francium most resembles caesium. No weighable quantity of the metal has ever been prepared. Altogether 31 isotopes of francium are known, all radioactive, and ranging from the alpha/inverse beta decaying francium-201 which has a halflife of just 48 milliseconds, to the beta decaying francium-231 with a halflife of 17 seconds. The longest lived isotope of francium, present on Earth in only trace amounts and the only isotope of francium found in nature, is francium-223 with a halflife of 21.8 minutes decaying by either alpha decay into the alpha/beta decaying astatine-219 which has a halflife of 4 seconds or by inverse beta decay into the alpha decaying radon-223 which has a halflife of 11 days. The francium-223 itself is produced in a minor branch-line of the radioactive decay of uranium-235 via the thorium-231, protactinium-231 and actinium-227, which are also present on Earth in just trace amounts. Claim to fame: Of the first 101 elements, francium, atomic number 87, is the most unstable, with most of its isotopes having extremely short halflives.
|
![]()