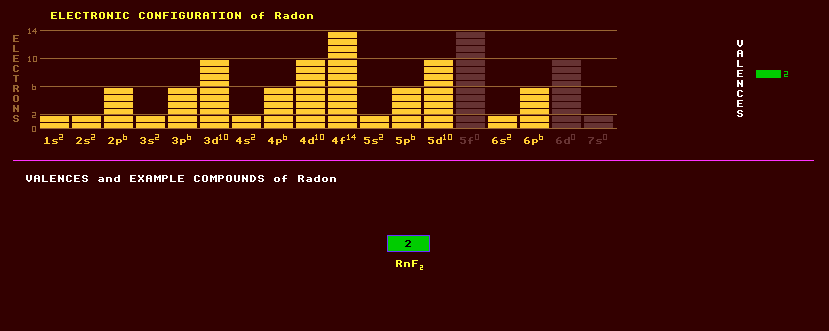

|
86 RADON Rn (named after radium)
A colourless, odourless noble monatomic radioactive gaseous element once thought to be completely inert due to its completed-octet electron shell, but now believed to form certain compounds with fluorine, like RnF2, but which are little studied because of the hazardous radiation which rapidly destroys any compounds formed.
Chemically, it should be like xenon. Radon, once called niton, is the most dense of the noble gases, and is nearly ten times heavier than air, but is very rare. At ordinary temperatures, radon is a colourless gas, but when cooled below its freezing point (-62ºC), it exhibits a brilliant phosphorescence which becomes yellow as the temperature is reduced, and orange-red at the temperature of liquid air. Isotope radon-222 is produced in the ground by the radioactive alpha-disintegration of radium-226, hence it was once called radium emanation. Natural radon consists of just traces of three radioactive isotopes, radon-219 (actinium emanation, or actinon) with a half-life of 4 seconds, radon-220 (thorium emanation, or thoron) of 56 seconds, and radon-226 (radium emanation, or radon), the longest lived isotope, with a half-life of 3.8 days. All three are alpha particle emitters which also give off gamma rays. Good ventilation should be provided where radium, thorium or actinium are stored to prevent build up of radon. Being dangerously radioactive and heavier than air, radon is a particular radiological hazard in certain areas of Britain where granite rocks, which contain traces of uranium and hence thorium and radium, predominate, as in Cornwall, the Lake District and certain parts of Derbyshire. It is here that radon gas given off by radium can accumulate in houses to dangerous levels, and good under-floor ventilation should be provided. Radon has been found to exceed the maximum permitted level in air (10-8 On average, every square mile of soil to a depth of six inches contains about 1 gram of radium, which slowly releases radon in tiny amounts to the atmosphere. Radon is present in some spring waters, such as those at Hot Springs, Arkansas, and in air at 1 part in 1021. Radon build up in uranium mines is a radiological hazard. The longest lived isotope of radon is the radioactive radon-222, which is present on Earth in trace amounts, and decays by alpha decay with a halflife of 3.82 days into the alpha decaying polonium-218 (halflife 1.6 seconds). Altogether, 31 isotopes of radon are known, all radioactive, and ranging from the alpha decaying radon-198 which has a halflife of just 50 milliseconds to the beta decaying radon-228 (halflife 1.08 minutes). Claim to fame: Radon has the lowest thermal conductivity of any element (36x10-6 W/cmºK) and is the heaviest gaseous element.
|
![]()