
|
85 ASTATINE At (Greek: astatos = unstable)
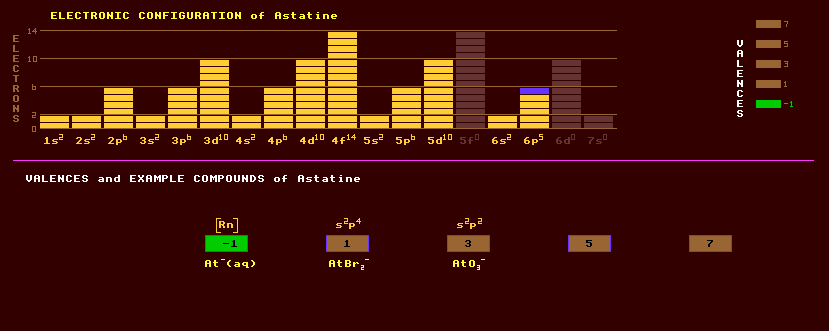
Astatine is the heaviest member of the halogen group of elements, group 17, along with fluorine, chlorine, bromine and iodine. Astatine does not occur naturally on Earth except in minute trace amounts because it is highly radioactive with no stable isotopes. Trace amounts of astatine-215 and astatine-219 exist naturally in secular equilibrium as a product of the decay of uranium-235; astatine-218 is similarly derived from uranium-238; and astatine-217 comes from uranium-233 and neptunium-237 which are produced by the interaction of thorium and uranium with neutrons emitted by the spontaneous fission of uranium. The total amount of astatine in the earths crust is less than 30 grams, possibly just a few milligrams. The longest lived isotope of astatine is astatine-210 with a halflife of 8.1 hours decaying by either alpha decay into the radioactive bismuth-206 or by inverse beta decay into the radioactive polonium-210. Astatine can be produced by bombarding a bismuth target with high energy alpha particles to obtain astatine-209, astatine-210 and astatine-211, which can be distilled from the target by heating. Much less than a microgram has been made to date (1978). Chemically, astatine is a halogen and very like iodine, but more metallic than iodine. Like iodine, it probably will accumulate in the thyroid gland. Inter-halogen compounds of AtI, AtBr and AtCl can be formed. The acid, HAt, has been detected, as has methyl astatide, CH3At. Altogether, 25 isotopes of astatine are known, all radioactive, ranging from the positron emitting astatine-196 with a halflife of 300 milliseconds to the electron emitting astatine-220, halflife 3.7 minutes. The longest lived isotope of astatine is astatine-210, with a halflife of 8 hours, decaying by either alpha decay into the inverse beta decaying bismuth-206 which has a halflife of 6 days or by inverse beta decay into the alpha decaying polonium-210 which has a halflife of 8 hours. Astatine 213 has a very short halflife of only 110 nanoseconds. Claim to fame: Astatine has the least lithospheric abundance of any natural element at 3 parts in 1026.
|
![]()