
|
84 POLONIUM Po (Poland)
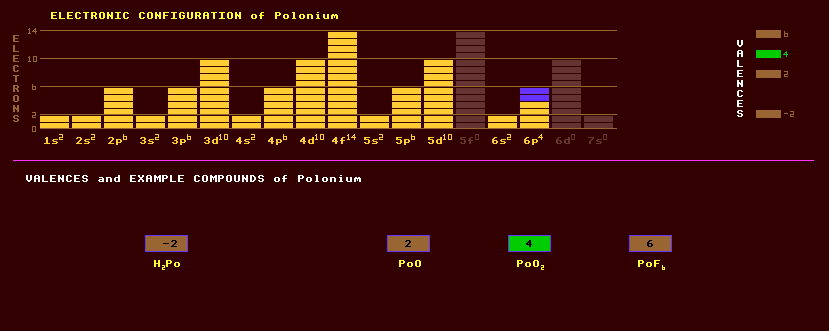
Polonium is a member of group 16 elements with oxygen, sulphur, selenium and tellurium. Polonium does not occur naturally on Earth except in trace amounts because it is highly radioactive with no stable isotopes. Uranium ores contain polonium-210 and polonium-218 at only 20 parts in 1012, formed in the decay series of uranium. Polonium is a volatile, low-melting point metal, half of which vaporises if kept at 55ºC for 45 hours. Two allotropic forms exist. Polonium-210 is now made in milligram quantities by bombarding bismuth-209 with neutrons creating bismuth-210, which decays with a halflife of 5 days into polonium-210. It is an alpha emitter, with a halflife of 138 days and emits 5000 times more alpha rays than does the same mass of radium. Discharging 140 watts per gram, a half a gram can reach 500 Celsius, making it useful as the energy source for thermo-electric generators in spacecraft. Polonium-210 emits a blue glow in air, caused by air ionization. Other isotopes can be prepared by the alpha, proton or deuteron bombardment of lead or bismuth in a cyclotron producing polonium-208, halflife 3 years, and polonium-209 with a halflife of 102 years, the longest lived isotope of polonium, and which decays by either alpha decay into the radioactive positron emitting lead-205 (halflife 15 Million years) or by inverse beta decay into stable bismuth-209. They are expensive to produce. Polonium exhibits four valences: -2, +2, the commonly expressed +3, and +4. Prepared compounds include polonium monoxide, PoO, the dioxide, PoO, the trioxide, PoO3; polonium dichloride, PoCl2, and dibromide, PoBr2; polonium tetrachloride, PoCl4, tetrabromide, PoBr4 and tetraiodide, PoI4; and polonium hexafluoride, PoF6; the hydride, H2Po; a compound with sodium, Na2Po; and the complex [PoI6]2-. Polonium is readily dissolved in dilute acids, but only slightly soluble in alkalis. Polonium salts of organic compounds char rapidly. Polonium-210 can be alloyed with beryllium to produce a neutron source. Polonium-210, carefully sealed in enclosures to protect humans, has been used in devices for neutralising static charges in textile mills and for brushes for removing dust from photographic films. Polonium-210 is exceedingly hazardous even in microgram quantities due to the complete absorption of its huge flux of alpha particles by human tissue. The maximum allowable ingestion of polonium-210 is just 7 picograms. By weight it is 3x1011 times more toxic than hydrocyanic acid. Polonium-210 was released into the atmosphere, contaminating surrounding countryside for miles, in the accidental atomic power station fire at Calder Hall in 1957, but this fact was kept secret for decades. It was being manufactured within the reactor for use as an atomic bomb trigger material. Polonium-210 has recently been found in trace amounts in the teeth of children who live near or within 10 kilometres of Motorways, and is a contaminant of leaded petrol. It has a halflife of just 138 days and is produced from lead-210, a beta emitter with a halflife of 23 years, which is itself derived from uranium-238 via uranium-234 with which it is in secular equilibrium. Polonium-218, a dangerous and prodigious alpha emitter with a halflife of just 3 minutes, can be deposited in the lungs by the alpha decay of any radon-222 gas that is incidentally inhaled. Radon gas is produced underground from the small amounts of uranium contained within granite rocks in certain areas, and can seep into homes. At present, 27 isotopes of polonium are known, all radioactive, all alpha emitters, ranging from the alpha decaying polonium-192 which has a halflife of 34 milli seconds to the alpha decaying polonium-218, with a halflife 3 minutes. The longest lived isotope of polonium is polonium-209 with a halflife of 102 years. Claim to fame: The first element identified by Madame Curie whilst investigating the radioactivity of pitchblende, a form of uraninite.
|
![]()